Enhanced Heterogeneous Fenton Degradation of Organic Dyes by Bimetallic Zirconia-Based Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural and Structural Characterization
2.2. Catalytic Activity
2.2.1. Monometallic Catalysts
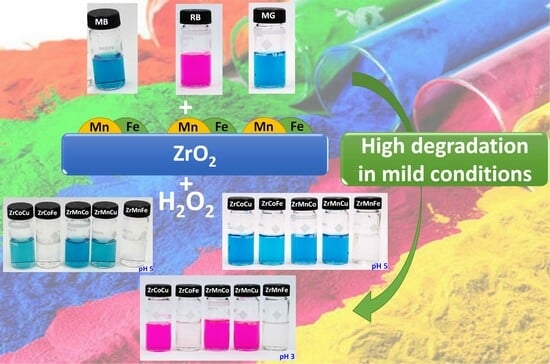
2.2.2. Bimetallic Catalysts
2.2.3. Comparison with the Literature Results
| Catalysts | [cat] (mg/L) | [H2O2] (mM) | [Pollutant] (mg/L) | [Cat]/[Pollutant] | T (°C) | pH | Time (min) | Degr. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ZrMnFe | 200 | 15 | 13.2 | 15 | 70 | 3 | 5 | 100 | This work |
| Cu–NaY | 300 | 175 | 50 | 6 | 60 | 6 | 60 | 100 | [65] |
| Cu-Cu2O | 300 | 1 | 3.65 | 82 | 60 | 3 | 30 | 100 | [67] |
| Zn/HAP/MgFe2O4 | 571 | 5 | 10 | 57 | 30 | 3–7 | 2 | 100 | [66] |
| ZrMnFe | 200 | 15 | 13.2 | 15 | 70 | 5 | 5 | 97 | This work |
| Fe3O4/SiO2 | 15 mg * | 50 μL * | 25 | n.a. | 30 | 6.7 | 30 | 96 | [68] |
| Diatomite/MnSiO3 | 400 | 30 | 500 | 0.8 | 30 | n.a. | 70 | 93 | [69] |
3. Materials and Methods
3.1. Materials
3.2. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, C. Biological effects of agriculturally derived surface water pollutants on aquatic systems—A review. J. Environ. Qual. 1993, 22, 402–408. [Google Scholar] [CrossRef]
- Gudmundsson, L.; Seneviratne, S.I.; Zhang, X. Anthropogenic climate change detected in European renewable freshwater resources. Nat. Clim. Chang. 2017, 7, 813–816. [Google Scholar] [CrossRef]
- Padowski, J.C.; Gorelick, S.M.; Thompson, B.H.; Rozelle, S.; Fendorf, S. Assessment of human–natural system characteristics influencing global freshwater supply vulnerability. Environ. Res. Lett. 2015, 10, 104014. [Google Scholar] [CrossRef]
- Rajmohan, K.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; van den Berg, S.J.; Dingemans, M.M.; Van den Brink, P.J. (Eco) toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of persistent organic pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw. Sustain. Dev. 2021, 13, 100575. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Al-Baldawi, I.A.; Mohammed, A.A.; Mutar, Z.H.; Abdullah, S.R.S.; Jasim, S.S.; Almansoory, A.F.; Ismail, N.I. Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis. J. Clean. Prod. 2021, 319, 128584. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Bhuta, H. Chapter 4—Advanced Treatment Technology and Strategy for Water and Wastewater Management. In Industrial Wastewater Treatment, Recycling and Reuse; Ranade, V.V., Bhandari, V.M., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 193–213. [Google Scholar]
- Bundschuh, M.; Pierstorf, R.; Schreiber, W.H.; Schulz, R. Positive Effects of Wastewater Ozonation Displayed by in Situ Bioassays in the Receiving Stream. Environ. Sci. Technol. 2011, 45, 3774–3780. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Henze, M.; Harremoes, P.; Jansen, J.L.; Arvin, E. Wastewater Treatment: Biological and Chemical Process; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Jeworski, M.; Heinzle, E. Combined chemical-biological treatment of wastewater containing refractory pollutants. Biotechnol. Annu. Rev. 2000, 6, 163–196. [Google Scholar]
- Farhadian, N.; Liu, S.; Asadi, A.; Shahlaei, M.; Moradi, S. Enhanced heterogeneous Fenton oxidation of organic pollutant via Fe-containing mesoporous silica composites: A review. J. Mol. Liq. 2021, 321, 114896. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424.24. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Briguglio, S.; Mattiussi, M.; Gelao, V.; Cabras, I.; Zorzenon, L.; Trovarelli, A.; Goi, D. Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J. Environ. Chem. Eng. 2020, 8, 103586. [Google Scholar] [CrossRef]
- Wols, B.; Hofman-Caris, C. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.; Kumar, A. Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochemistry 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.-K.; Kim, S.-J.; Kim, B.-H.; Yoon, H.-S.; Jung, S.-C. Rapid degradation of methyl orange using hybrid advanced oxidation process and its synergistic effect. J. Ind. Eng. Chem. 2016, 35, 205–210. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H.; Zen, N. Hybrid advanced oxidation process (HAOP) as highly efficient and powerful treatment for complete demineralization of antibiotics. Sep. Purif. Technol. 2020, 241, 116728. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Zhang, M.-h.; Dong, H.; Zhao, L.; Wang, D.-x.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Comuzzi, C.; Baderna, D.; Zuccaccia, D.; Trovarelli, A.; Goi, D. Abatement of the ecotoxicological risk of landfill leachate by heterogeneous Fenton-like oxidation. Environ. Sci. Pollut. Res. 2023, 30, 21025–21032. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Trovarelli, A.; Goi, D. Removal of organics from landfill leachate by heterogeneous fenton-like oxidation over copper-based catalyst. Catalysts 2022, 12, 338. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Trovarelli, A.; Goi, D. Heterogeneous Fenton-like oxidation of ibuprofen over zirconia-supported iron and copper catalysts: Effect of process variables. J. Water Process Eng. 2021, 44, 102343. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D.; Trovarelli, A. Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization. Catalysts 2021, 11, 1383. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Meena, S.; Vaya, D.; Das, B. Photocatalytic degradation of Malachite Green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 2016, 39, 1735–1743. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Shirsath, D.S.; Patil, P.S.; Shrivastava, V.S. Adsorptive and photocatalytic removal of carcinogenic methylene blue dye by SnO2 nanorods: An equilibrium, kinetic and thermodynamics exploration. SN Appl. Sci. 2020, 2, 822. [Google Scholar] [CrossRef]
- Teng, X.; Li, J.; Wang, Z.; Wei, Z.; Chen, C.; Du, K.; Zhao, C.; Yang, G.; Li, Y. Performance and mechanism of methylene blue degradation by an electrochemical process. RSC Adv. 2020, 10, 24712–24720. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Magnacca, G.; Cerrato, G.; Morterra, C.; Signoretto, M.; Somma, F.; Pinna, F. Structural and Surface Characterization of Pure and Sulfated Iron Oxides. Chem. Mater. 2003, 15, 675–687. [Google Scholar] [CrossRef]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO Supported on Tetragonal ZrO2 Catalysts for N2O Decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, Z.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Novel Mn–Zr Mixed Oxide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408.52. [Google Scholar] [CrossRef]
- Yang, B.; Tian, Z.; Zhang, L.; Guo, Y.; Yan, S. Enhanced heterogeneous Fenton degradation of Methylene Blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J. Water Process Eng. 2015, 5, 101–111. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Y.; Li, X.; Sun, T.; Xu, Y. Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4. RSC Adv. 2018, 8, 1071–1077. [Google Scholar] [CrossRef]
- Wu, X.; Xia, F.; Nan, Z. Facile synthesis of double-mesoporous-shelled hollow spheres of Cu–CuFe2O4/SiO2 composite as excellent Fenton catalyst. Mater. Chem. Phys. 2020, 242, 122490. [Google Scholar] [CrossRef]
- Li, D.; Yang, T.; Li, Y.; Liu, Z.; Jiao, W. Facile and green synthesis of highly dispersed tar-based heterogeneous Fenton catalytic nanoparticles for the degradation of methylene blue. J. Clean. Prod. 2020, 246, 119033. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Cai, Y.F.; Zhang, Q.J.; Wang, H.; Liu, Y.L. Fe/Acid-montmorillonite as effective Fenton-like catalyst for the removal of methylene blue. J. Chem. Technol. Biotechnol. 2022, 97, 3163–3171. [Google Scholar] [CrossRef]
- Ahmed, H.R.; Mustafa, F.S.; Aziz, K.H.H.; Hinder, S.J. Utilizing sequence transformation of selective copper metal as an efficient heterogeneous Fenton-like catalyst for the degradation of aqueous methylene blue. React. Kinet. Mech. Catal. 2024, 137, 115–132. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, Y.; Zhang, H.; Fan, H.; Li, Y.; Yan, Z.; Zhang, W.-H. Synthesis of magnetic biochar-supported Fe-Cu bimetallic catalyst from pulp and paper mill wastes for the Fenton-like removal of rhodamine B dye. Chem. Eng. J. 2023, 477, 146823. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, P.; Ding, D.; Yang, Z.; Wang, W.; Xin, H.; Xu, J.; Han, Y.-F. Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl. Catal. B Environ. 2019, 258, 117985. [Google Scholar] [CrossRef]
- Zhu, G.; Xiong, S.; Shi, C.; Jin, Y.; Ge, M. Fe-Cu@ γ-Al2O3 microspheres as a heterogeneous Fenton-like catalyst for degrading polyvinyl alcohol, Rhodamine-B, and Reactive Red X-3B. J. Mol. Liq. 2022, 365, 120118. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Shi, S.; Liu, E.; Li, T.; Wei, S.; Li, Y.; Li, Y.; Sun, G.; Zhao, Z. Efficient and stable iron-copper montmorillonite heterogeneous Fenton catalyst for removing Rhodamine B. Chem. Phys. Lett. 2021, 776, 138673. [Google Scholar] [CrossRef]
- Zhang, N.; Tsang, E.P.; Chen, J.; Fang, Z.; Zhao, D. Critical role of oxygen vacancies in heterogeneous Fenton oxidation over ceria-based catalysts. J. Colloid Interface Sci. 2020, 558, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; You, J.-A.; Chen, C.; Zhang, H.; Ji, X.Z.; Li, C.; Yang, Y.; Xu, N.; Huang, J. Manganese functionalized mesoporous molecular sieves Ti-HMS as a Fenton-like catalyst for dyes wastewater purification by advanced oxidation processes. J. Environ. Chem. Eng. 2016, 4, 4653–4660. [Google Scholar] [CrossRef]
- Fathima, N.N.; Aravindhan, R.; Rao, J.R.; Nair, B.U. Dye house wastewater treatment through advanced oxidation process using Cu-exchanged Y zeolite: A heterogeneous catalytic approach. Chemosphere 2008, 70, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Dhar, S.S. Remarkable catalytic degradation of malachite green by zinc supported on hydroxyapatite encapsulated magnesium ferrite (Zn/HAP/MgFe2O4) magnetic novel nanocomposite. J. Mater. Sci. 2020, 55, 4592–4606. [Google Scholar] [CrossRef]
- Meriem, H.; Souad, D.; Lakhdar, T. Synthesis of copper with sodium ascorbate and its application in malachite green discoloration. J. Environ. Chem. Eng. 2020, 8, 104457. [Google Scholar] [CrossRef]
- Mahmud, N.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.H.; Mohammad, A.W. Effective Heterogeneous Fenton-Like degradation of Malachite Green Dye Using the Core-Shell Fe3O4@ SiO2 Nano-Catalyst. ChemistrySelect 2021, 6, 865–875. [Google Scholar] [CrossRef]
- Jiang, D.B.; Yuan, Y.; Zhao, D.; Tao, K.; Xu, X.; Zhang, Y.X. Facile synthesis of three-dimensional diatomite/manganese silicate nanosheet composites for enhanced Fenton-like catalytic degradation of malachite green dye. J. Nanoparticle Res. 2018, 20, 123. [Google Scholar] [CrossRef]







| Name | Nominal Composition | Surface Area (m2/g) | H2 Consumption (mmol/g) |
|---|---|---|---|
| ZrCo | Co(2.5%)/ZrO2 | 53 | 0.44 |
| ZrCu | Cu(2.5%)/ZrO2 | 56 | 0.40 |
| ZrFe | Fe(2.5%)/ZrO2 | 55 | 0.43 |
| ZrMn | Mn(2.5%)/ZrO2 | 53 | 0.42 |
| ZrCoCu | Co(2.5%)- Cu(2.5%)/ZrO2 | 46 | 0.41 |
| ZrCoFe | Co(2.5%)- Fe(2.5%)/ZrO2 | 46 | 0.60 |
| ZrMnCo | Mn(2.5%)- Co(2.5%)/ZrO2 | 52 | 0.57 |
| ZrMnCu | Mn(2.5%)- Cu(2.5%)/ZrO2 | 54 | 0.40 |
| ZrMnFe | Mn(2.5%)- Fe(2.5%)/ZrO2 | 52 | 0.71 |
| Catalysts | [Cat] (mg/L) | [H2O2] (mM) | [Pollutant] (mg/L) | [Cat]/[Pollutant] | T (°C) | pH | Time (min) | Degr. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ZrMnFe | 200 | 15 | 16.6 | 12 | 70 | 5 | 5 | 100 | This work |
| FeNi/C-300 | 1000 | 100 | 30 | 33 | 45 | 7 | 60 | 98 | [55] |
| Fe/Acid-MMT | 750 | 0.85 | 50 | 15 | 50 | 3 | 30 | 98 | [56] |
| Fe/Acid-MMT | 1000 | 0.85 | 50 | 20 | 50 | 6 | 300 | 98 | [56] |
| Cu-CuFe2O4/SiO2 | 200 | 197 | 50 | 4 | 25 | 7 | 120 | 98 | [54] |
| Fe0-Fe3O4-RGO | 100 | 0.8 | 50 | 2 | 25 | 3 | 60 | 98 | [52] |
| Cu-CB | 750 | 3 | 20 | 38 | 30 | 6.7 | 30 | 97 | [57] |
| ZrCoFe | 200 | 15 | 16.6 | 12 | 70 | 5 | 5 | 97 | This work |
| FeII@MIL-100(Fe) | 1000 | 40 | 500 | 2 | 25 | 3 | 300 | 90 | [58] |
| CuFe2O4 | 100 | 0.5 | 50 | 2 | 25 | 3 | 25 | 74 | [53] |
| Catalysts | [Cat] (mg/L) | [H2O2] (mM) | [Pollutant] (mg/L) | [Cat]/[Pollutant] | T (°C) | pH | Time (min) | Degr. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FeCu@BC600-2 | 200 | 1 | 10 | 20 | 30 | 3 | 60 | 100 | [59] |
| FeCu@BC600-2 | 250 | 25 | 10 | 25 | 30 | 7 | 360 | 100 | [59] |
| Cu/SiO2 | 500 | 29 | 10 | 50 | 60 | n.a. | 60 | 100 | [60] |
| Fe-Cu/γ-Al2O3 | 2200 | 309 | 200 | 11 | 70 | 7 | 210 | 100 | [61] |
| ZrMnFe | 200 | 15 | 14.2 | 14 | 70 | 3 | 30 | 98 | This work |
| Fe/Cu-MMT | 1500 | 5 | 100 | 15 | 25 | 7 | 90 | 98 | [62] |
| ZrCoFe | 200 | 15 | 14 | 14 | 70 | 3 | 30 | 96 | This work |
| FeCeOx | 1500 | 80 | 100 | 15 | 35 | 3 | 150 | 91 | [63] |
| FeCeOx | 1500 | 80 | 100 | 15 | 35 | 5 | 150 | 88 | [63] |
| Mn/Ti-HMS | 1000 | 10 | 24 | 42 | 25 | 7 | 100 | 50 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aneggi, E.; Hussain, S.; Baratta, W.; Zuccaccia, D.; Goi, D. Enhanced Heterogeneous Fenton Degradation of Organic Dyes by Bimetallic Zirconia-Based Catalysts. Molecules 2024, 29, 2074. https://doi.org/10.3390/molecules29092074
Aneggi E, Hussain S, Baratta W, Zuccaccia D, Goi D. Enhanced Heterogeneous Fenton Degradation of Organic Dyes by Bimetallic Zirconia-Based Catalysts. Molecules. 2024; 29(9):2074. https://doi.org/10.3390/molecules29092074
Chicago/Turabian StyleAneggi, Eleonora, Sajid Hussain, Walter Baratta, Daniele Zuccaccia, and Daniele Goi. 2024. "Enhanced Heterogeneous Fenton Degradation of Organic Dyes by Bimetallic Zirconia-Based Catalysts" Molecules 29, no. 9: 2074. https://doi.org/10.3390/molecules29092074