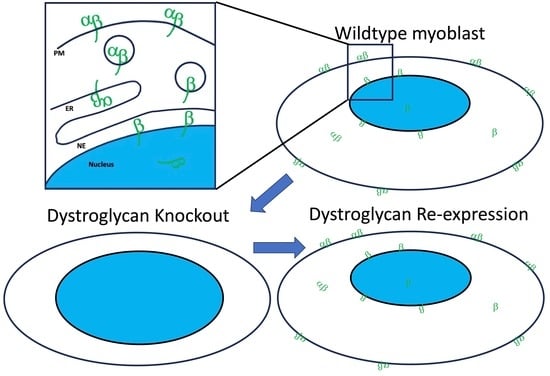
The Role of β-Dystroglycan in Nuclear Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Generation of DAG1 KO Myoblasts
2.2. Cellular Fractionation
2.3. Cell Cycle Synchronisation by Serum Starvation
2.4. SDS-PAGE and Western Blotting
2.5. PCR and Agarose Gel Electrophoresis
2.6. RNA Extraction and cDNA Generation
2.7. Molecular Cloning
2.8. TOPO-TA Cloning for DAG1 KO Characterisation
2.9. Plasmid Transfection Procedures
2.10. Immunofluorescence Staining and Microscopy
2.11. Atomic Force Microscopy
2.12. Live Cell Imaging for Transwell Assays
2.13. Image Analysis
2.13.1. Nuclear Morphology
2.13.2. Cell Migration Analyses
3. Results
3.1. Generation of Dystroglycan-Knockout Human Myoblasts Using CRISPR/Cas9
3.2. Establishing Specific Dystroglycan Antibodies and Localisation to the Nucleus
3.3. Generation of an Epitope-Tagged Version of DAG1 to Establish Subcellular Compartmentalisation
3.4. DAG1 KO Cells Do Not Display a Defect in Nuclear Morphology
3.5. DAG1 KO Nuclei Are Not More Sensitive to Mechanical Strain than WT Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, C.J.; Winder, S.J. Dystroglycan versatility in cell adhesion: A tale of multiple motifs. Cell Commun. Signal. 2010, 8, 3. [Google Scholar] [CrossRef]
- Fuentes-Mera, L.; Rodríguez-Muñoz, R.; González-Ramírez, R.; García-Sierra, F.; González, E.; Mornet, D.; Cisneros, B. Characterization of a novel Dp71 dystrophin-associated protein complex (DAPC) present in the nucleus of HeLa cells: Members of the nuclear DAPC associate with the nuclear matrix. Exp. Cell Res. 2006, 312, 3023–3035. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Morales-Lázaro, S.; Tapia-Ramírez, V.; Mornet, D.; Cisneros, B. Nuclear and nuclear envelope localization of dystrophin Dp71 and dystrophin-associated proteins (DAPs) in the C2C12 muscle cells: DAPs nuclear localization is modulated during myogenesis. J. Cell. Biochem. 2008, 105, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Lara-Chacón, B.; de León, M.B.; Leocadio, D.; Gómez, P.; Fuentes-Mera, L.; Martínez-Vieyra, I.; Ortega, A.; Jans, D.A.; Cisneros, B. Characterization of an importin in α/β-recognized nuclear localization signal in β-dystroglycan. J. Cell. Biochem. 2010, 110, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Gracida-Jiménez, V.; Mondragón-González, R.; Vélez-Aguilera, G.; Vásquez-Limeta, A.; Laredo-Cisneros, M.S.; Gómez-López, J.d.D.; Vaca, L.; Gourlay, S.C.; Jacobs, L.A.; Winder, S.J.; et al. Retrograde trafficking of β-dystroglycan from the plasma membrane to the nucleus. Sci. Rep. 2017, 7, 9906. [Google Scholar] [CrossRef]
- Oppizzi, M.L.; Akhavan, A.; Singh, M.; Fata, J.E.; Muschler, J.L. Nuclear translocation of β-dystroglycan reveals a distinctive trafficking pattern of autoproteolyzed mucins. Traffic 2008, 9, 2063–2072. [Google Scholar] [CrossRef]
- Vásquez-Limeta, A.; Wagstaff, K.M.; Ortega, A.; Crouch, D.H.; Jans, D.A.; Cisneros, B. Nuclear import of β-dystroglycan is facilitated by ezrin-mediated cytoskeleton reorganization. PLoS ONE 2014, 9, e90629. [Google Scholar] [CrossRef]
- Leocadio, D.; Mitchell, A.; Winder, S.J. γ-Secretase Dependent Nuclear Targeting of Dystroglycan. J. Cell. Biochem. 2016, 117, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.; Parkin, C.A.; Juusola, M.; Winder, S.J. The proteasomal inhibitor MG132 prevents muscular dystrophy in zebrafish. PLoS Curr. 2011, 3, RRN1286. [Google Scholar] [CrossRef]
- Miller, G.; Moore, C.J.; Terry, R.; La Riviere, T.; Mitchell, A.; Piggott, R.; Dear, T.N.; Wells, D.J.; Winder, S.J. Preventing phosphorylation of dystroglycan ameliorates the dystrophic phenotype in mdx mouse. Hum. Mol. Genet. 2012, 21, 4508–4520. [Google Scholar] [CrossRef]
- Sotgia, F.; Bonuccelli, G.; Bedford, M.; Brancaccio, A.; Mayer, U.; Wilson, M.T.; Campos-Gonzalez, R.; Brooks, J.W.; Sudol, M.; Lisanti, M.P. Localization of phospho-β-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochemistry 2003, 42, 7110–7123. [Google Scholar] [CrossRef]
- Bozzi, M.; Inzitari, R.; Sbardell, D.; Monaco, S.; Pavoni, E.; Gioia, M.; Marini, S.; Morlacchi, S.; Sciandra, F.; Castagnola, M.; et al. Enzymatic processing of β-dystroglycan recombinant ectodomain by MMP-9: Identification of the main cleavage site. IUBMB Life 2009, 61, 1143–1152. [Google Scholar] [CrossRef]
- Michaluk, P.; Kolodziej, L.; Mioduszewska, B.; Wilczynski, G.M.; Dzwonek, J.; Jaworski, J.; Gorecki, D.C.; Ottersen, O.P.; Kaczmarek, L. β-Dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 2007, 282, 16036–16041. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Inzitari, R.; Iavarone, F.; Gioia, M.; Marini, S.; Sciandra, F.; Castagnola, M.; Steen, P.E.V.D.; Opdenakker, G.; Giardina, B.; et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse β-dystroglycan. IUBMB Life 2012, 64, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Losasso, C.; Di Tommaso, F.; Sgambato, A.; Ardito, R.; Cittadini, A.; Giardina, B.; Petrucci, T.C.; Brancaccio, A. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000, 484, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.; Mitchell, A.; Down, J.M.; Jacobs, L.A.; Hamdy, F.C.; Eaton, C.; Rosario, D.J.; Cross, S.S.; Winder, S.J. Nuclear targeting of dystroglycan promotes the expression of androgen regulated transcription factors in prostate cancer. Sci. Rep. 2013, 3, 2792. [Google Scholar] [CrossRef]
- Singh, J.; Itahana, Y.; Knight-Krajewski, S.; Kanagawa, M.; Campbell, K.P.; Bissell, M.J.; Muschler, J. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 2004, 64, 6152–6159. [Google Scholar] [CrossRef]
- Riemersma, M.; Mandel, H.; van Beusekom, E.; Gazzoli, I.; Roscioli, T.; Eran, A.; Gershoni-Baruch, R.; Gershoni, M.; Pietrokovski, S.; Vissers, L.E.; et al. Absence of α- and β-dystroglycan is associated with Walker-Warburg syndrome. Neurology 2015, 84, 2177–2182. [Google Scholar] [CrossRef]
- Williamson, R.A.; Henry, M.D.; Daniels, K.J.; Hrstka, R.F.; Lee, J.C.; Sunada, Y.; Ibraghimov-Beskrovnaya, O.; Campbell, K.P. Dystroglycan is essential for early embryonic development: Disruption of Reichert’s membrane in DAG1-null mice. Hum. Mol. Genet. 1997, 6, 831–841. [Google Scholar] [CrossRef]
- Dinçer, P.; Balcı, B.; Yuva, Y.; Talim, B.; Brockington, M.; Dinçel, D.; Torelli, S.; Brown, S.; Kale, G.; Haliloglu, G.; et al. A novel form of recessive limb girdle muscular dystrophy with mental retardation and abnormal expression of α-dystroglycan. Neuromuscul. Disord. 2003, 13, 771–778. [Google Scholar] [CrossRef]
- Dong, M.; Noguchi, S.; Endo, Y.; Hayashi, Y.K.; Yoshida, S.; Nonaka, I.; Nishino, I. DAG1 mutations associated with asymptomatic hyperCKemia and hypoglycosylation of α-dystroglycan. Neurology 2015, 84, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Balci-Hayta, B.; Yoshida-Moriguchi, T.; Kanagawa, M.; de Bernabé, D.B.-V.; Gündeşli, H.; Willer, T.; Satz, J.S.; Crawford, R.W.; Burden, S.J.; et al. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N. Engl. J. Med. 2011, 364, 939–946. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Volpato, C.B.; Motta, B.M.; Blankenburg, H.; Picard, A.; Pramstaller, P.; Casella, M.; Rauhe, W.; Pompilio, G.; Meraviglia, V.; et al. Exploring digenic inheritance in arrhythmogenic cardiomyopathy. BMC Med. Genet. 2017, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Sasaoka, T.; Noguchi, S.; Nishino, I.; Tanaka, T. Cys669–Cys713 disulfide bridge formation is a key to dystroglycan cleavage and subunit association. Genes Cells 2007, 12, 75–88. [Google Scholar] [CrossRef]
- Mamchaoui, K.; Trollet, C.; Bigot, A.; Negroni, E.; Chaouch, S.; Wolff, A.; Kandalla, P.K.; Marie, S.; Di Santo, J.; Guily, J.L.S.; et al. Immortalized pathological human myoblasts: Towards a universal tool for the study of neuromuscular disorders. Skelet. Muscle 2011, 1, 34. [Google Scholar] [CrossRef]
- Martínez-Vieyra, I.A.; Vásquez-Limeta, A.; González-Ramírez, R.; Morales-Lázaro, S.L.; Mondragón, M.; Mondragón, R.; Ortega, A.; Winder, S.J.; Cisneros, B. A role for β-dystroglycan in the organization and structure of the nucleus in myoblasts. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 698–711. [Google Scholar] [CrossRef]
- Kirby, T.J.; Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 2018, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Tamiello, C.; Kamps, M.A.; Wijngaard, A.v.D.; Verstraeten, V.L.R.M.; Baaijens, F.P.; Broers, J.L.; Bouten, C.C. Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus 2013, 4, 61–73. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Science, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Earle, A.J.; Kirby, T.J.; Fedorchak, G.R.; Isermann, P.; Patel, J.; Iruvanti, S.; Moore, S.A.; Bonne, G.; Wallrath, L.L.; Lammerding, J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2020, 19, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Thompson, O.; Moore, C.J.; Hussain, S.-A.; Kleino, I.; Peckham, M.; Hohenester, E.; Ayscough, K.R.; Saksela, K.; Winder, S.J. Modulation of cell spreading and cell-substrate adhesion dynamics by dystroglycan. J. Cell Sci. 2010, 123, 118–127. [Google Scholar] [CrossRef]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nature 2014, 16, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Shree, N.; Manu, E.; Guzniczak, E.; Otto, O. Cardiomyocyte mechanodynamics under conditions of actin remodelling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Winder, S.J. The inside and out of dystroglycan post-translational modification. Neuromuscul. Disord. 2012, 22, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, P.; Columbaro, M.; Mura, I.; Capanni, C.; Lattanzi, G.; Maraldi, N.M.; de Barnabè, D.B.-V.; van Bokoven, H.; Squarzoni, S.; Merlini, L. Extracellular matrix and nuclear abnormalities in skeletal muscle of a patient with Walker–Warburg syndrome caused by POMT1 mutation. Biochim. Biophys. Acta—Mol. Basis Dis. 2003, 1638, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi-Ikeda, M.; Morioka, I.; Iijima, K.; Toda, T. Mechanistic aspects of the formation of α-dystroglycan and therapeutic research for the treatment of α-dystroglycanopathy: A review. Mol. Asp. Med. 2016, 51, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Crivelli, S.N.; Singh, M.; Lingappa, V.R.; Muschler, J.L. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 2008, 22, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Sciandra, F.; Angelucci, E.; Altieri, F.; Ricci, D.; Hübner, W.; Petrucci, T.C.; Giardina, B.; Brancaccio, A.; Bozzi, M. Dystroglycan is associated to the disulfide isomerase ERp57. Exp. Cell Res. 2012, 318, 2460–2469. [Google Scholar] [CrossRef]
- Signorino, G.; Covaceuszach, S.; Bozzi, M.; Hübner, W.; Mönkemöller, V.; Konarev, P.V.; Cassetta, A.; Brancaccio, A.; Sciandra, F. A dystroglycan mutation (p.Cys667Phe) associated to muscle-eye-brain disease with multicystic leucodystrophy results in ER-retention of the mutant protein. Hum. Mutat. 2018, 39, 266–280. [Google Scholar] [CrossRef]
- Gómez-Monsivais, W.L.; Monterrubio-Ledezma, F.; Huerta-Cantillo, J.; Mondragon-Gonzalez, R.; Alamillo-Iniesta, A.; García-Aguirre, I.; Azuara-Medina, P.M.; Arguello-García, R.; Rivera-Monroy, J.E.; Holaska, J.M.; et al. The Molecular Basis and Biologic Significance of the β-Dystroglycan-Emerin Interaction. Int. J. Mol. Sci. 2020, 21, 5944. [Google Scholar] [CrossRef]
- Vélez-Aguilera, G.; Gómez-López, J.d.D.; Jiménez-Gutiérrez, G.E.; Vásquez-Limeta, A.; Laredo-Cisneros, M.S.; Gómez, P.; Winder, S.J.; Cisneros, B. Control of nuclear β-dystroglycan content is crucial for the maintenance of nuclear envelope integrity and function. Biochim. Biophys. Acta—Mol. Cell Res. 2018, 1865, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Azuara-Medina, P.M.; Sandoval-Duarte, A.M.; Morales-Lázaro, S.L.; Modragón-González, R.; Vélez-Aguilera, G.; Gómez-López, J.d.D.; Jiménez-Gutiérrez, G.E.; Tiburcio-Félix, R.; Martínez-Vieyra, I.; Suárez-Sánchez, R.; et al. The intracellular domain of β-dystroglycan mediates the nucleolar stress response by suppressing UBF transcriptional activity. Cell Death Dis. 2019, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Mathew, G.; Jiang, T.; Hamdy, F.; Cross, S.; Eaton, C.; Winder, S. Dystroglycan function is a novel determinant of tumor growth and behavior in prostate cancer. Prostate 2013, 73, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Spence, H.J.; Cameron, J.M.; Jess, T.; Ilsley, J.L.; Winder, S.J. Direct interaction of β-dystroglycan with F-actin. Biochem. J. 2003, 375 (Pt 2), 329–337. [Google Scholar] [CrossRef]
- Jacobs, L. Dystroglycan in the Nucleus and the Cell Cycle; The University of Sheffield: Sheffield, UK, 2017. [Google Scholar]
- Côté, P.D.; Moukhles, H.; Carbonetto, S. Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin α7B expression and caveolin-3 distribution. J. Biol. Chem. 2002, 277, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gutierrez, G.E.; Mondragon-Gonzalez, R.; Soto-Ponce, L.A.; Gómez-Monsiváis, W.L.; García-Aguirre, I.; Pacheco-Rivera, R.A.; Suárez-Sánchez, R.; Brancaccio, A.; Magaña, J.J.; Perlingeiro, R.C.; et al. Loss of dystroglycan drives cellular senescence via defective mitosis-mediated genomic instability. Int. J. Mol. Sci. 2020, 21, 4961. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ren, R.; Liu, Z.; Song, M.; Li, J.; Wu, Z.; Ren, X.; Fu, L.; Li, W.; Zhang, W.; et al. Stabilizing heterochromatin by DGCR8 alleviates senescence and osteoarthritis. Nat. Commun. 2019, 10, 3329. [Google Scholar] [CrossRef]
- Dreesen, O.; Ong, P.F.; Chojnowski, A.; Colman, A. The contrasting roles of lamin B1 in cellular aging and human disease. Nucleus 2013, 4, 283–290. [Google Scholar] [CrossRef]
- Freund, A.; Laberge, R.-M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef]
- Sadaie, M.; Dillon, C.; Narita, M.; Young, A.R.J.; Cairney, C.J.; Godwin, L.S.; Torrance, C.J.; Bennett, D.C.; Keith, W.N.; Narita, M. Cell-based screen for altered nuclear phenotypes reveals senescence progression in polyploid cells after Aurora kinase B inhibition. Mol. Biol. Cell 2015, 26, 2971–2985. [Google Scholar] [CrossRef]
- Salunkhe, S.; Mishra, S.V.; Nair, J.; Shah, S.; Gardi, N.; Thorat, R.; Sarkar, D.; Rajendra, J.; Kaur, E.; Dutt, S. Nuclear localization of p65 reverses therapy-induced senescence. J. Cell Sci. 2021, 134, jcs253203. [Google Scholar] [CrossRef]
- Shah, P.P.; Donahue, G.; Otte, G.L.; Capell, B.C.; Nelson, D.M.; Cao, K.; Aggarwala, V.; Cruickshanks, H.A.; Rai, T.S.; McBryan, T.; et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, L.; Wang, K.; Wu, D.; Trappio, M.; Witting, C.; Cao, K. Loss of H3K9me3 correlates with ATM activation and histone H2AX phosphorylation deficiencies in Hutchinson-Gilford progeria syndrome. PLoS ONE 2016, 11, e0167454. [Google Scholar] [CrossRef] [PubMed]
- Ilsley, J.L.; Sudol, M.; Winder, S.J. The interaction of dystrophin with β-dystroglycan is regulated by tyrosine phosphorylation. Cell. Signal. 2001, 13, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Pereboev, A.V.; Ahmed, N.; Man, N.T.; Morris, G.E. Epitopes in the interacting regions of β-dystroglycan (PPxY motif) and dystrophin (WW domain). Biochim. Biophys. Acta—Gen. Subj. 2001, 1527, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Rivier, F.; Robert, A.; Hugon, G.; Bonet-Kerrache, A.; Nigro, V.; Fehrentz, J.-A.; Martinez, J.; Mornet, D. Dystrophin and utrophin complexed with different associated proteins in cardiac Purkinje fibres. Histochem. J. 1999, 31, 425–432. [Google Scholar] [CrossRef]







| Clone | PCR Product (bp) | β-Dystroglycan Protein | Mutation Induced | |
|---|---|---|---|---|
| gRNA_001 | gRNA_002 | |||
| WT | ~840 | Expressed | N/A | |
| 10D | ~840 | Expressed | N/A | |
| 1.B8 | ~840 | Expressed | N/A | |
| 1.B9 | ~840 | Expressed | N/A | |
| 11A | 3 bands | Not detected | 1 bp deletion | 5 bp deletion |
| 1.B6 | ~630 | Not detected | Excision: CT insertion | |
| 1.C2 | ~630 | Not detected | Excision: C insertion | |
| 1.G4 | ~840 | Not detected, predicted aa79 * | 1 bp deletion | 1 bp deletion |
| 1.G7 | ~630 | Not detected | Excision: C insertion | |
| 2.C7 | ~630 | Not detected | Excision: C insertion | |
| 2.C9 | ~630 | Not detected | Excision: C insertion | |
| 2.D8 | ~630 | Not detected | Heterozygous excision: C insertion and end joining. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, M.; Stevenson, B.; Jacobs, L.A.; Leocadio Victoria, D.; Cisneros, B.; Hobbs, J.K.; Stewart, C.L.; Winder, S.J. The Role of β-Dystroglycan in Nuclear Dynamics. Cells 2024, 13, 431. https://doi.org/10.3390/cells13050431
Cook M, Stevenson B, Jacobs LA, Leocadio Victoria D, Cisneros B, Hobbs JK, Stewart CL, Winder SJ. The Role of β-Dystroglycan in Nuclear Dynamics. Cells. 2024; 13(5):431. https://doi.org/10.3390/cells13050431
Chicago/Turabian StyleCook, Matthew, Ben Stevenson, Laura A. Jacobs, Daniel Leocadio Victoria, Bulmaro Cisneros, Jamie K. Hobbs, Colin L. Stewart, and Steve J. Winder. 2024. "The Role of β-Dystroglycan in Nuclear Dynamics" Cells 13, no. 5: 431. https://doi.org/10.3390/cells13050431