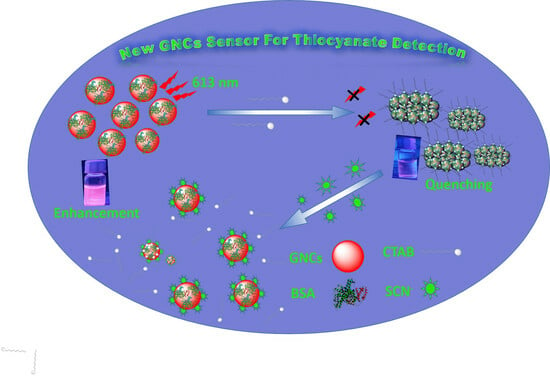
Design a Friendly Nanoscale Chemical Sensor Based on Gold Nanoclusters for Detecting Thiocyanate Ions in Food Industry Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Synthesis of CTAB-Gold Nanoclusters (CTAB-GNCs)
2.4. Synthesis of Gold Nanoclusters (GNCs)
2.5. Detection of SCN− Ions Using CTAB-GNCs
2.6. Real Sample Preparation
3. Results
3.1. Synthesis and Optical Properties of CTAB-GNCs
3.2. Studying the Optimal Concentration of CTAB
3.3. GNCS and CTAB-GNCs Characterization
3.4. SCN− Detection Mechanism Based on CTAB-GNCs
3.5. Interference and Selectivity of CTAB-GNCs toward SCN−
3.6. Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Mo, R.; Wang, L.; Zhou, C.; Hong, P.; Li, C. Surface enhanced Raman spectroscopy detection of sodium thiocyanate in milk based on the aggregation of Ag nanoparticles. Sensors 2019, 19, 1363. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Cui, X.; Lu, Y.; Yu, M.; Fei, Q.; Feng, G.; Shan, H.; Huan, Y. Colorimetric determination of cysteine based on Au@ Pt nanoparticles as oxidase mimetics with enhanced selectivity. Microchim. Acta 2022, 189, 13. [Google Scholar] [CrossRef] [PubMed]
- Willemin, M.E.; Lumen, A. Characterization of the modes of action and dose-response relationship for thiocyanate on the thyroid hormone levels in rats using a computational approach. Toxicol. Appl. Pharmacol. 2019, 365, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Conner, G.E.; Wijkstrom-Frei, C.; Randell, S.H.; Fernandez, V.E.; Salathe, M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007, 581, 271–278. [Google Scholar] [PubMed]
- Banfi, B. A novel host defense system of airways is defective in cystic fibrosis: Update. Am. J. Respir. Crit. Care Med. 2007, 175, 967. [Google Scholar] [PubMed]
- Xu, Y.; Szép, S.; Lu, Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. USA 2009, 106, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, F.; Zhang, J. Voltammetric behaviors and determination of thiocyanate on multiwalled carbon nanotubes-cetyltrimethylammonium bromide modified electrode. Electroanalysis 2018, 30, 2413–2420. [Google Scholar] [CrossRef]
- Akiyama, H.; Matsuoka, H.; Okuyama, T.; Higashi, K.; Toida, T.; Komatsu, H.; Sugita-Konishi, Y.; Kobori, S.; Kodama, Y.; Yoshida, M.; et al. The acute encephalopathy induced by intake of sugihiratake mushroom in the patients with renal damage might be associated with the intoxication of cyanide and thiocyanate. Food Saf. 2015, 3, 16–29. [Google Scholar]
- Jiang, B.; Zhong, S.; Yu, H.; Chen, P.; Li, B.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Aqueous Two-Phase System–Ion Chromatography for Determination of Thiocyanate in Raw Milk. Separations 2021, 8, 212. [Google Scholar] [CrossRef]
- Destanoğlu, O.; Gümüş Yılmaz, G. Determination of cyanide, thiocyanate, cyanate, hexavalent chromium, and metal cyanide complexes in various mixtures by ion chromatography with conductivity detection. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 465–474. [Google Scholar]
- Lobo-Júnior, E.O.; LS Chagas, C.; Coltro, W.K. Determination of inorganic cations in biological fluids using a hybrid capillary electrophoresis device coupled with contactless conductivity detection. J. Sep. Sci. 2018, 41, 3310–3317. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Kagaya, S.; Gemmei-Ide, M.; Cattrall, R.W.; Kolev, S.D. The use of a polymer inclusion membrane as a sorbent for online preconcentration in the flow injection determination of thiocyanate impurity in ammonium sulfate fertilizer. Talanta 2014, 129, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, P.C.; Wan, Y.Q.; Wu, F.Y. Colorimetric detection of thiocyanate based on anti-aggregation of gold nanoparticles in the presence of cetyltrimethyl ammonium bromide. Sens. Actuators B Chem. 2016, 222, 790–796. [Google Scholar] [CrossRef]
- Peng, C.F.; Pan, N.; Zhi-Juan, Q.; Wei, X.L.; Shao, G. Colorimetric detection of thiocyanate based on inhibiting the catalytic activity of cystine-capped core-shell Au@ Pt nanocatalysts. Talanta 2017, 175, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, E.; Servarayan, K.L.; Vasantha, V.S. Optical detection of thiocyanate in human saliva based on the colorimetric response of (2-(2-hydroxyphenyl)-1H-benzo [d] imidazol-5-yl)(phenyl) methanone (HBPM)/Co2+ ions conjugate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120423. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Sherazee, M.; Srinivasan, S.; Rajabzadeh, A.R. Positively charged gold quantum dots: An nanozymatic “off-on” sensor for thiocyanate detection. Foods 2022, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Wirojsaengthong, S.; Aryuwananon, D.; Aeungmaitrepirom, W.; Pulpoka, B.; Tuntulani, T. A colorimetric paper-based optode sensor for highly sensitive and selective determination of thiocyanate in urine sample using cobalt porphyrin derivative. Talanta 2021, 231, 122371. [Google Scholar] [CrossRef]
- Bai, X.R.; Zhang, L.; Ren, J.Q.; Shen, A.G.; Hu, J.M. The small silver nanoparticle-assisted homogeneous sensing of thiocyanate ions with an ultra-wide window based on surface-enhanced Raman-extinction spectroscopy. Anal. Methods 2021, 13, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Zheng, Y.; Huang, Z.; Cheng, Y.; Ye, J.; Chu, Q.; Huang, D. Study on the potential application of salivary inorganic anions in clinical diagnosis by capillary electrophoresis coupled with contactless conductivity detection. J. Chromatogr. B 2016, 1014, 70–74. [Google Scholar] [CrossRef]
- Ali, R.; Alminderej, F.M.; Saleh, S.M. A simple, quantitative method for spectroscopic detection of metformin using gold nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118744. [Google Scholar] [CrossRef]
- Saleh, S.M.; Alminderej, F.M.; Ali, R.; Abdallah, O.I. Optical sensor film for metribuzin pesticide detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117971. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Hegazy, M.E.F.; Alminderej, F.M.; Mohamed, T.A. The natural compound chrysosplenol-D is a novel, ultrasensitive optical sensor for detection of Cu(II). J. Mol. Liq. 2020, 302, 112558. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Alminderej, F.; Ali, I.A. Ultrasensitive optical chemosensor for Cu(II) detection. Int. J. Anal. Chem. 2019, 2019, 7381046. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Ali, R.; Ali, I.A. A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Almotiri, M.K.; Ali, R. Green synthesis of highly luminescent gold nanoclusters and their application in sensing Cu(II) and Hg(II). J. Photochem. Photobiol. A Chem. 2022, 426, 113719. [Google Scholar] [CrossRef]
- Zuber, G.; Weiss, E.; Chiper, M. Biocompatible gold nanoclusters: Synthetic strategies and biomedical prospects. Nanotechnology 2019, 30, 352001. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Saleh, S.M.; Aly, S.M. Fluorescent gold nanoclusters as pH sensors for the pH 5 to 9 range and for imaging of blood cell pH values. Microchim. Acta 2017, 184, 3309–3315. [Google Scholar] [CrossRef]
- Matus, M.F.; Häkkinen, H. Atomically precise gold nanoclusters: Towards an optimal biocompatible system from a theoretical–experimental strategy. Small 2021, 17, 2005499. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Cheng, T.M.; Chu, H.L.; Tan, S.H.; Kuo, J.C.; Hsu, P.H.; Su, C.Y.; Chen, H.M.; Lee, C.M.; Kuo, T.R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Ali, R.; Alfeneekh, B.; Chigurupati, S.; Saleh, S.M. Green synthesis of pregabalin–stabilized gold nanoclusters and their applications in sensing and drug release. Arch. Pharm. 2022, 355, 2100426. [Google Scholar] [CrossRef]
- Halawa, M.I.; Lai, J.; Xu, G. Gold nanoclusters: Synthetic strategies and recent advances in fluorescent sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horiz. 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Liao, F.; Dang, Q.; Shao, M. Fluorescent-stable and water-soluble two-component-modified silicon quantum dots and their application for bioimaging. J. Lumin. 2019, 215, 116644. [Google Scholar] [CrossRef]
- Borse, S.; Murthy, Z.V.P.; Park, T.J.; Kailasa, S.K. Pepsin mediated synthesis of blue fluorescent copper nanoclusters for sensing of flutamide and chloramphenicol drugs. Microchem. J. 2021, 164, 105947. [Google Scholar] [CrossRef]
- Yang, L.; Hou, P.; Wei, J.; Li, B.; Gao, A.; Yuan, Z. Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review. Molecules 2024, 29, 1574. [Google Scholar] [CrossRef]
- Bai, Y.; Shu, T.; Su, L.; Zhang, X. Fluorescent gold nanoclusters for biosensor and bioimaging application. Crystals 2020, 10, 357. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, W.J.; Kim, K.O.; Park, J.H.; Lee, S.K. Performance improvement of a glucose sensor based on fiber optic localized surface plasmon resonance and anti-aggregation of the non-enzymatic receptor. J. Alloys Compd. 2021, 884, 161140. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Li, J.; Chen, L. Nanomaterial-based optical sensors for mercury ions. TrAC Trends Anal. Chem. 2016, 82, 175–190. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Golmohammadi, H.; Abbasi-Moayed, S.; Nejad, M.A.F.; Fahimi-Kashani, N.; Jafarinejad, S.; Shahrajabian, M.; Hormozi-Nezhad, M.R. Nanoparticle-based optical sensor arrays. Nanoscale 2017, 9, 16546–16563. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. TrAC Trends Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. In Colloidal Synthesis of Plasmonic Nanometals; Jenny Stanford Publishing: Singapore, 2020; pp. 197–220. [Google Scholar]
- Abdullah, A.; Altaf, M.; Khan, H.I.; Khan, G.A.; Khan, W.; Ali, A.; Bhatti, A.S.; Khan, S.U.; Ahmed, W. Facile room temperature synthesis of multifunctional CTAB coated gold nanoparticles. Chem. Phys. 2018, 510, 30–36. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Niu, H.; Zhang, X.; Cai, Y. One-step synthesis of silver/dopamine nanoparticles and visual detection of melamine in raw milk. Analyst 2011, 136, 4192–4196. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Song, A.; Hao, J. Effect of Cationic Surfactants with Different Counterions on the Growth of Au Nanoclusters. Langmuir 2018, 34, 6138–6146. [Google Scholar] [CrossRef]
- Bakshi, M.S. How surfactants control crystal growth of nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133. [Google Scholar] [CrossRef]
- Dai, C.; Yang, C.; Yan, X. Self-quenched gold nanoclusters for turn-on fluorescence imaging of intracellular glutathione. Nano Res. 2018, 11, 2488–2497. [Google Scholar] [CrossRef]
- Murthy, A.K.; Stover, R.J.; Borwankar, A.U.; Nie, G.D.; Gourisankar, S.; Truskett, T.M.; Sokolov, K.V.; Johnston, K.P. Equilibrium gold nanoclusters quenched with biodegradable polymers. ACS Nano 2013, 7, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Guo, W.; Qi, Y.; Wang, J.; Ma, X.; Yu, D. Synergistic effects of gold nanocages in hyperthermia and radiotherapy treatment. Nanoscale Res Lett. 2016, 11, 279. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Sher, F. Smart nano-architectures as potential sensing tools for detecting heavy metal ions in aqueous matrices. Trends Environ. Anal. Chem. 2022, 36, e00179. [Google Scholar] [CrossRef]
- Zhang, Y.; Mollick, S.; Tricarico, M.; Ye, J.; Sherman, D.A.; Tan, J.C. Turn-on fluorescence chemical sensing through transformation of self-trapped exciton states at room temperature. ACS Sens. 2022, 7, 2338–2344. [Google Scholar] [CrossRef]
- Ali, R.; Alminderej, F.M.; Messaoudi, S.; Saleh, S.M. Ratiometric ultrasensitive optical chemisensor film based antibiotic drug for Al (III) and Cu (II) detection. Talanta 2021, 221, 121412. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O.; Miu, I. Development and validation of a spectrometric method for Cd and Pb determination in zeolites and safety evaluation. Molecules 2020, 25, 2591. [Google Scholar] [CrossRef] [PubMed]
- Klymus, K.E.; Merkes, C.M.; Allison, M.J.; Goldberg, C.S.; Helbing, C.C.; Hunter, M.E.; Jackson, C.A.; Lance, R.F.; Mangan, A.M.; Monroe, E.M.; et al. Reporting the limits of detection and quantification for environmental DNA assays. Environ. DNA 2020, 2, 271–282. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, T.; Lin, Y.W. Fluorescence sensing of mercury (ii) and melamine in aqueous solutions through microwave-assisted synthesis of egg-white-protected gold nanoclusters. Anal. Methods 2018, 10, 1624–1632. [Google Scholar] [CrossRef]









| Samples | Added SCN− (nM) | Found (nM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Liquid milk | 20.0 | 21.2 | 3.6 | 106 |
| 60.0 | 64.2 | 3.1 | 107 | |
| 100.0 | 103.2 | 2.2 | 103 | |
| 140 | 137.3 | 1.7 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R.; Saleh, S.M. Design a Friendly Nanoscale Chemical Sensor Based on Gold Nanoclusters for Detecting Thiocyanate Ions in Food Industry Applications. Biosensors 2024, 14, 223. https://doi.org/10.3390/bios14050223
Ali R, Saleh SM. Design a Friendly Nanoscale Chemical Sensor Based on Gold Nanoclusters for Detecting Thiocyanate Ions in Food Industry Applications. Biosensors. 2024; 14(5):223. https://doi.org/10.3390/bios14050223
Chicago/Turabian StyleAli, Reham, and Sayed M. Saleh. 2024. "Design a Friendly Nanoscale Chemical Sensor Based on Gold Nanoclusters for Detecting Thiocyanate Ions in Food Industry Applications" Biosensors 14, no. 5: 223. https://doi.org/10.3390/bios14050223