Current Status of Fertility Preservation in Pediatric Oncology Patients
Abstract
:1. Introduction
2. Methods and Results
2.1. Present Situation
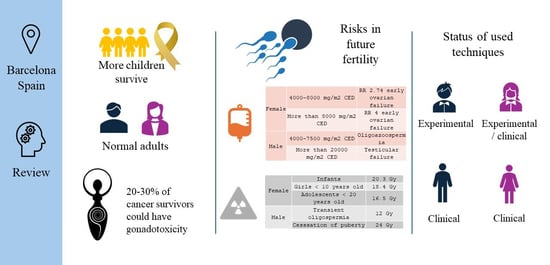
2.2. Risks to Future Fertility
2.3. Chemotherapy
2.4. Radiation Therapy
2.5. Other Interventions
2.6. Indications for Fertility Preservation
2.7. Used Techniques
2.7.1. Prepubertal Children
2.7.2. Adolescents and Young Adult Men
2.7.3. Prepubertal Girls
2.7.4. Adolescent and Young Adult Women
2.7.5. Preventive Measures and Advocating for Gonadal Preservation in Benign Tumors
2.8. Ethical Considerations
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiller, C. Epidemiology of Pediatric Cancer. In Pediatric Surgical Oncology, 1st ed.; Losty, P., LaQuaglia, M., Sarnacki, S., Füchs, J., Taguchi, T., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 3–9. [Google Scholar]
- Nieto, C.A.; Romaguera, P.E.; López, M.A.; Poveda, V.S.; Cebolla, P.S.; Reines, B.M.; Bonet, P.R. Cáncer Infantil en España. Estadísticas 1980–2021. Registro Español de Tumores Infantiles (RETI-SEHOP). 2022. Available online: http://www.uv.es/rnti (accessed on 1 March 2024).
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71 Pt B, 1017–1033. [Google Scholar] [CrossRef]
- O’Leary, M.; Krailo, M.; Anderson, J.R.; Reaman, G.H. Progress in Childhood Cancer: 50 Years of Research Collaboration, a Report From the Children’s Oncology Group. Semin Oncol. 2008, 35, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Radauer-Plank, A.C.; Diesch-Furlanetto, T.; Schneider, M.; Sommerhäuser, G.; Friedrich, L.A.; Salow, V.; Dülberg, J.; Diepold, M.; Rovó, A.; Njue, L.M.; et al. Desire for biological parenthood and patient counseling on the risk of infertility among adolescents and adults with hemoglobinopathies. Pediatr. Blood Cancer 2023, 70, e30359. [Google Scholar] [CrossRef] [PubMed]
- Brungardt, J.G.; Burns, K.C.; Dasgupta, R. Fertility preservation in children and young adults with cancer. Curr. Opin. Pediatr. 2022, 34, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Capito, C.; Labiad, C.; Helfre, S.; Bolle, S.; Grynberg, M.; Sarnacki, S. Fertility Considerations and the Pediatric Cancer Patient. In Pediatric Surgical Oncology, 1st ed.; Losty, P., LaQuaglia, M., Sarnacki, S., Füchs, J., Taguchi, T., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 361–366. [Google Scholar]
- Mulder, R.L.; Font-Gonzalez, A.; van Dulmen-den Broeder, E.; Quinn, G.P.; Ginsberg, J.P.; Loeffen, E.A.H.; Hudson, M.M.; Burns, K.C.; van Santen, H.M.; Berger, C.; et al. Communication and ethical considerations for fertility preservation for patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e68–e80. [Google Scholar] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.L.; Font-Gonzalez, A.; Hudson, M.M.; van Santen, H.M.; Loeffen, E.A.H.; Burns, K.C.; Quinn, G.P.; van Dulmen-den Broeder, E.; Byrne, J.; Haupt, R.; et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e45–e56. [Google Scholar] [CrossRef]
- Martinez, F.; International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group. Update on fertility preservation from the Barcelona International Society for Fertility Preservation–ESHRE–ASRM 2015 expert meeting: Indications, results and future perspectives. Fertil. Steril. 2017, 108, 407–415.e11. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Matsumoto, K.; Boku, N.; Fujii, N.; Tsuchida, Y.; Furui, T.; Harada, M.; Kanda, Y.; Kawai, A.; Miyachi, M.; et al. Indications for fertility preservation not included in the 2017 Japan Society of Clinical Oncology Guideline for Fertility Preservation in Pediatric, Adolescent, and Young Adult Patients treated with gonadal toxicity, including benign diseases. Int. J. Clin. Oncol. 2022, 27, 301–309. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Anastacio, A.; Vonheim, E.; Deen, S.; Malmros, J.; Borgström, B. Fertility preservation for young adults, adolescents, and children with cancer. Ups. J. Med. Sci. 2020, 125, 112–120. [Google Scholar] [CrossRef]
- Suzuki, N. Clinical Practice Guidelines for Fertility Preservation in Pediatric, Adolescent, and Young Adults with Cancer. Int. J. Clin. Oncol. 2019, 24, 20–27. [Google Scholar] [CrossRef]
- Robson, D.; Phua, C.; Howard, R.; Marren, A. Fertility preservation in oncology patients: A literature review examining current fertility preservation techniques and access to oncofertility services in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Duffin, K.; Howie, R.; Kelsey, T.W.; Wallace, H.B.; Anderson, R.A. Long-Term Follow-Up to Assess Criteria for Ovarian Tissue Cryopreservation for Fertility Preservation in Young Women and Girls with Cancer. Hum. Reprod. 2023, 38, 1076–1085. Available online: https://academic.oup.com/humrep/advance-article/doi/10.1093/humrep/dead060/7100840 (accessed on 29 February 2024). [CrossRef] [PubMed]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reprod. Anniv. Rev. 2018, 156, F11–F27. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J.; Cacciottola, L. Fertility Preservation: The Challenge of Freezing and Transplanting Ovarian Tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef]
- Moussaoui, D.; Surbone, A.; Adam, C.; Diesch-Furlanetto, T.; Girardin, C.; Bénard, J.; Vidal, I.; Bernard, F.; Busiah, K.; Bouthors, T.; et al. Testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys: A 6 year experience from a Swiss multi-center network. Front. Pediatr. 2022, 6, 909000. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Almeida-Santos, A.T.; Melo, C.; Rama, A.C.R. Decision on Fertility Preservation in Cancer Patients: Development of Information Materials for Healthcare Professionals. J. Adolesc. Young Adult Oncol. 2017, 6, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Márquez-Vega, C.; Rodríguez-Lescure, Á.; Rovirosa, Á.; Vázquez, L.; Zeberio-Etxetxipia, I.; Andrés, M.; Bassas, L.; Ceballos-Garcia, E.; Domingo, J.; et al. Multidisciplinary consensus on the criteria for fertility preservation in cancer patients. Clin. Transl. Oncol. 2022, 24, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Gertosio, C.; Magistrali, M.; Musso, P.; Meazza, C.; Bozzola, M. Fertility Preservation in Pediatric Oncology Patients: New Perspectives. J. Adolesc. Young Adult Oncol. 2018, 7, 263–269. [Google Scholar] [CrossRef]
- Appiah, L.C. Fertility Preservation for Adolescents Receiving Cancer Therapies. Clin. Obs. Gynecol. 2020, 63, 574–587. [Google Scholar] [CrossRef]
- Burns, K.C.; Hoefgen, H.; Strine, A.; Dasgupta, R. Fertility preservation options in pediatric and adolescent patients with cancer. Cancer 2018, 124, 1867–1876. [Google Scholar] [CrossRef]
- Green, D.M.; Nolan, V.G.; Goodman, P.J.; Whitton, J.A.; Srivastava, D.; Leisenring, W.M.; Neglia, J.P.; Sklar, C.A.; Kaste, S.C.; Hudson, M.M.; et al. The Cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the childhood cancer Survivor Study. Pediatr. Blood Cancer 2014, 61, 53–67. [Google Scholar] [CrossRef]
- Pampanini, V.; Hassan, J.; Oliver, E.; Stukenborg, J.B.; Damdimopoulou, P.; Jahnukainen, K. Fertility Preservation for Prepubertal Patients at Risk of Infertility: Present Status and Future Perspectives. Horm. Res. Paediatr. 2021, 93, 599–608. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Winship, A.L.; Hutt, K.J. Do cancer therapies damage the uterus and compromise fertility? Hum. Reprod. Update 2020, 26, 161–173. [Google Scholar] [CrossRef]
- Corkum, K.S.; Rhee, D.S.; Wafford, Q.E.; Demeestere, I.; Dasgupta, R.; Baertschiger, R.; Malek, M.M.; Aldrink, J.H.; Heaton, T.E.; Weil, B.R.; et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: A systematic review. J. Pediatr. Surg. 2019, 54, 2200–2209. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Dong, L.; Gul, M.; Fode, M.; Hildorf, S.; Thorup, J.; Hoffmann, E.; Cortes, D.; Fedder, J.; Andersen, C.Y.; et al. Fertility preservation in boys facing gonadotoxic cancer therapy. Nat. Rev. Urol. 2022, 19, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.N. Fertility preservation in the pediatric cancer patient. Curr. Opin. Urol. 2019, 29, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Hildorf, S.; Dong, L.; Thorup, J.; Hoffmann, E.R.; Jensen, C.F.S.; Sønksen, J.; Cortes, D.; Fedder, J.; Andersen, C.Y.; et al. Review of injection techniques for spermatogonial stem cell transplantation. Hum. Reprod. Update 2020, 26, 368–391. [Google Scholar] [CrossRef]
- Faes, K.; Lahoutte, T.; Hoorens, A.; Tournaye, H.; Goossens, E. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod. Biomed. Online 2017, 34, 291–297. [Google Scholar] [CrossRef]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef]
- Kanbar, M.; Delwiche, G.; Wyns, C. Fertility preservation for prepubertal boys: Are we ready for autologous grafting of cryopreserved immature testicular tissue? Ann. Endocrinol. 2022, 83, 210–217. [Google Scholar] [CrossRef]
- Richer, G.; Baert, Y.; Goossens, E. In-vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Gholami, K.; Solhjoo, S.; Aghamir, S.M.K. Application of Tissue-Specific Extracellular Matrix in Tissue Engineering: Focus on Male Fertility Preservation. Reprod. Sci. 2022, 29, 3091–3099. [Google Scholar] [CrossRef]
- Lautz, T.B.; Burns, K.; Rowell, E.E. Fertility Considerations in Pediatric and Adolescent Patients Undergoing Cancer Therapy. Surg. Oncol. Clin. N. Am. 2021, 30, 401–415. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Sanfilippo, J.S.; Valli-Pulaski, H. Female fertility preservation in the pediatric and adolescent cancer patient population. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 147–157. [Google Scholar] [CrossRef]
- Poirot, C.; Fortin, A.; Lacorte, J.M.; Akakpo, J.P.; Genestie, C.; Vernant, J.P.; Brice, P.; Morice, P.; Leblanc, T.; Gabarre, J.; et al. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum. Reprod. 2019, 34, 1083–1094. [Google Scholar] [CrossRef]
- Poirot, C.; Fortin, A.; Dhédin, N.; Brice, P.; Socié, G.; Lacorte, J.M.; Akakpo, J.P.; Genestie, C.; Vernant, J.P.; Leblanc, T.; et al. Post-transplant outcome of ovarian tissue cryopreserved after chemotherapy in hematologic malignancies. Haematologica 2019, 104, e360–e363. [Google Scholar] [CrossRef]
- Chevillon, F.; Clappier, E.; Arfeuille, C.; Cayuela, J.M.; Dalle, J.H.; Kim, R.; Caye-Eude, A.; Chalas, C.; Abdo, C.; Drouineaud, V.; et al. Minimal residual disease quantification in ovarian tissuecollected from patients in complete remission ofacute leukemia. Blood 2021, 137, 1697–1701. [Google Scholar] [CrossRef]
- Fortin, A.; Azaïs, H.; Uzan, C.; Lefebvre, G.; Canlorbe, G.; Poirot, C. Laparoscopic ovarian tissue harvesting and orthotopic ovarian cortex grafting for fertility preservation: Less is more. Fertil. Steril. 2019, 111, 408–410. [Google Scholar] [CrossRef]
- de Lambert, G.; Poirot, C.; Guérin, F.; Brugières, L.; Martelli, H. Preservation of future fertility in pediatric patients with cancer. J. Visc. Surg. 2018, 155, S41–S46. [Google Scholar] [CrossRef]
- Irene Su, H.; Lee, Y.T.; Barr, R. Oncofertility: Meeting the fertility goals of adolescents and young adults with cancer. Cancer J. 2018, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Finlayson, C.; Rowell, E.E.; Gosiengfiao, Y.; Pavone, M.E.; Lockart, B.; Orwig, K.E.; Brannigan, R.E.; Woodruff, T.K. Fertility Preservation for Pediatric Patients: Current State and Future Possibilities. J. Urol. 2017, 198, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Appiah, L.C.; Anazodo, A.; Burns, K.C.; Gomez-Lobo, V.; Hoefgen, H.R.; Jaworek Frias, O.; Laronda, M.M.; Levine, J.; Meacham, L.R.; et al. Development of a Pediatric Fertility Preservation Program: A Report From the Pediatric Initiative Network of the Oncofertility Consortium. J. Adolesc. Health 2019, 64, 563–573. [Google Scholar] [CrossRef] [PubMed]
- El Alaoui-Lasmaili, K.; Nguyen-Thi, P.L.; Demogeot, N.; Lighezzolo-Alnot, J.; Gross, M.J.; Mansuy, L.; Chastagner, P.; Koscinski, I. Fertility discussions and concerns in childhood cancer survivors, a systematic review for updated practice. Cancer Med. 2022, 12, 6023–6039. [Google Scholar] [CrossRef] [PubMed]
- Vindrola-Padros, C.; Dyer, K.E.; Cyrus, J.; Lubker, I.M. Healthcare professionals’ views on discussing fertility preservation with young cancer patients: A mixed method systematic review of the literature. Psycho-Oncol. 2017, 26, 4–14. [Google Scholar] [CrossRef]
- Ramstein, J.J.; Halpern, J.; Gadzinski, A.J.; Brannigan, R.E.; Smith, J.F. Ethical, moral, and theological insights into advances in male pediatric and adolescent fertility preservation. Andrology 2017, 5, 631–639. [Google Scholar] [CrossRef]
| Female | 4000–8000 mg/m2 CED | RR 2.74 early ovarian failure |
| More than 8000 mg/m2 CED | RR 4 early ovarian failure | |
| Male | 4000–7500 mg/m2 CED | Oligozoospermia |
| More than 20,000 mg/m2 CED | Testicular failure | |
| RR: relative risk | ||
| Infants | 20.3 Gy |
| Girls < 10 years old | 18.4 Gy |
| Adolescents < 20 years old | 16.5 Gy |
| High risk | >80% | Total body irradiation (TBI), pelvic or testicular radiotherapy, chemotherapy before HSCT, Hodgkin’s or non-Hodgkin’s lymphoma treated with alkylating agents and/or TBI, stage IV soft tissue sarcomas, metastatic Ewing sarcoma. |
| Intermediate risk | 40–80% | Myeloblastic acute leukemia, neuroblastoma, stage II–III soft tissue sarcomas, osteosarcoma, non-metastatic Ewing sarcoma, hepatoblastoma, non-Hodgkin’s lymphoma, CNS tumors with radiation dose >24 Gy. |
| Low risk | <40% | Wilms’s tumor, lymphoblastic acute leukemia, stage I soft tissue sarcomas, retinoblastoma, CNS tumors with radiation dose <24 Gy or operated only, non-irradiated germ cell tumors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasten González, A.; Salvador Alarcón, C.; Mora, J.; Martín Gimenez, M.P.; Carrasco Torrents, R.; Krauel, L. Current Status of Fertility Preservation in Pediatric Oncology Patients. Children 2024, 11, 537. https://doi.org/10.3390/children11050537
Pasten González A, Salvador Alarcón C, Mora J, Martín Gimenez MP, Carrasco Torrents R, Krauel L. Current Status of Fertility Preservation in Pediatric Oncology Patients. Children. 2024; 11(5):537. https://doi.org/10.3390/children11050537
Chicago/Turabian StylePasten González, Albert, Cristina Salvador Alarcón, Jaume Mora, Marta P. Martín Gimenez, Rosalia Carrasco Torrents, and Lucas Krauel. 2024. "Current Status of Fertility Preservation in Pediatric Oncology Patients" Children 11, no. 5: 537. https://doi.org/10.3390/children11050537