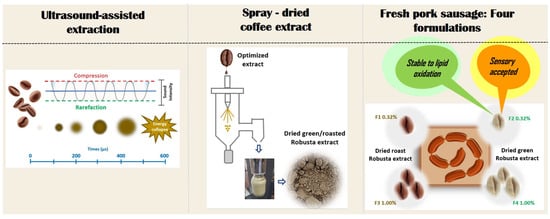
Coffee Extract as a Natural Antioxidant in Fresh Pork Sausage—A Model Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Ultrasound-Assisted Extraction (UAE) and Optimization Procedure
2.3. Analysis of Bioactive Compounds and Antioxidant Activity
2.4. Response Surface Analysis, Desirability and Experimental Validation
2.5. Different Coffee Samples Extraction under Optimized Conditions and Spray-Drying Process
2.6. Application of Dry Coffee Extracts as Natural Antioxidants in a Fresh Pork Sausage
2.7. Physicochemical, Sensory Acceptance, and Instrumental Evaluation
2.8. Data Analysis
3. Results and Discussion
3.1. Obtaining Coffee Extract for Use as a Natural Antioxidant: CCRD Approach
3.2. Application of Coffee Extract as a Natural Antioxidant in Fresh Pork Sausage: Oxidative Stability, Physicochemical, and Sensory Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- ICO. Annual Review 2017/18. Available online: https://www.ico.org/documents/cy2018-19/annual-review-2017-18-e.pdf (accessed on 4 April 2024).
- ICO. Coffee Report and Outlook: 2023. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 4 April 2024).
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- ICO Coffee Development Report 2021: “The Future of Coffee: Investing in Youth for a Resilient and Sustainable Coffee Sector”. Available online: https://www.ico.org/documents/cy2022-23/ed-2427e-cdr-2021.pdf (accessed on 4 April 2024).
- Cid, M.C.; de Peña, M.P. Coffee: Analysis and Composition. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2015; pp. 225–231. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A Study of Chemical Composition, Antioxidants, and Volatile Compounds in Roasted Arabic Coffee: Chemical Composition, Antioxidants and Volatile Compounds in Roasted Arabic Coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.C.M.; Oda, F.B.; Almeida-Cincotto, M.G.J.; Davanço, M.G.; Chiari-Andréo, B.G.; Cicarelli, R.M.B.; Peccinini, R.G.; Zocolo, G.J.; Ribeiro, P.R.V.; Corrêa, M.A.; et al. Green Coffee Seed Residue: A Sustainable Source of Antioxidant Compounds. Food Chem. 2018, 246, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Acquaticci, L.; Angeloni, S.; Cela, N.; Galgano, F.; Vittori, S.; Caprioli, G.; Condelli, N. Impact of Coffee Species, Post-Harvesting Treatments and Roasting Conditions on Coffee Quality and Safety Related Compounds. Food Control 2023, 149, 109714. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.d.T. Roasting Process Affects Differently the Bioactive Compounds and the Antioxidant Activity of Arabica and Robusta Coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of Ultrasound-Assisted Extraction to Obtain Mycosterols from Agaricus bisporus L. by Response Surface Methodology and Comparison with Conventional Soxhlet Extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem 2021, 70, 105325. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Comparison and Optimization of Conventional and Ultrasound Assisted Extraction for Bioactive Compounds and Antioxidant Activity from Agro-Industrial Acerola (Malpighia emarginata DC) Residue. LWT—Food Sci. Technol. 2017, 85, 158–169. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.; Sun, D.; Tiwari, B.K. Ultrasound-assisted Extraction (UAE) of Bioactive Compounds from Coffee Silverskin: Impact on Phenolic Content, Antioxidant Activity, and Morphological Characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, D.; Eun, J.; Moon, S. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of Alternative Solvents to Extract Biologically Active Compounds from Green Coffee Beans and Its Residue from the Oil Industry. Food Bioprod. Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Bravo, J.; Monente, C.; Juániz, I.; De Peña, M.P.; Cid, C. Influence of Extraction Process on Antioxidant Capacity of Spent Coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Barros Neto, B.; Scarmínio, I.S.; Bruns, R.E. Como Fazer Experimentos, 4th ed.; Bookman: Porto Alegre, Brazil, 2010; pp. 265–298. [Google Scholar]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of Coffee Antioxidants: Impact of Brewing Time and Method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Marcucci, C.T.; De Toledo Benassi, M.; Almeida, M.B.; Nixdorf, S.L. Teores de Trigonelina, Ácido 5-Cafeoilquínico, Cafeína e Melanoidinas Em Cafés Solúveis Comerciais Brasileiros. Quim. Nova 2013, 36, 544–548. [Google Scholar] [CrossRef]
- Dias, R.; Benassi, M. Discrimination between Arabica and Robusta Coffees Using Hydrosoluble Compounds: Is the Efficiency of the Parameters Dependent on the Roast Degree? Beverages 2015, 1, 127–139. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Biasuz, T.; De Conti, A.J.; Viegas, M.C.; Corso, M.P.; Benassi, M.d.T. Sensory Characterization and Acceptance of Coffee Brews of C. Arabica and C. Canephora Blended with Steamed Defective Coffee. Food Res. Int. 2019, 124, 234–238. [Google Scholar] [CrossRef]
- Corso, M.P.; Vignoli, J.A.; Benassi, M.d.T. Development of an Instant Coffee Enriched with Chlorogenic Acids. J. Food Sci. Technol. 2016, 53, 1380–1388. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.d.T. Antioxidant Activity of Roasted and Instant Coffees: Standardization and Validation of Methodologies. Coffee Sci. 2012, 7, 68–75. [Google Scholar]
- Instrução Normativa SDA n° 4 de 31/03/2000. Available online: https://www.legisweb.com.br/legislacao/?id=453107#:~:text=Aprova%20os%20regulamentos%20t%C3%A9cnicos%20de,que%20lhe%20confere%20o%20art (accessed on 21 April 2024).
- Viera, V.B.; Piovesan, N.; Moro, K.I.B.; Rodrigues, A.S.; Scapin, G.; Rosa, C.S.; Kubota, E.H. Preparation and microbiological analysis of Tuscan sausage with added propolis extract. Food Sci. Technol. 2016, 36 (Suppl. S1), 37–41. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Pearson, A.M.; Dugan, L.R. Chemistry of the 2-thiobarbituric acid test for determination of oxidative rancidity in foods—II Formation of the TBA—Malonaldehyde complex without acid-heat treatment. J. Sci. Food Agric. 1964, 15, 602. [Google Scholar] [CrossRef]
- Crackel, R.H.L.; Gray, J.I.; Booren, A.M.; Pearson, A.M.; Bucley, D.J. Effect of Antioxidants on Lipid Stability in Restructured Beef Steaks. J. Food Sci. 1988, 53, 656–657. [Google Scholar] [CrossRef]
- Almeida, M.B.; Benassi, M.D.T. Atividade Antioxidante e Estimativa Do Teor de Melanoidinas Em Cafés Torrados Comerciais. Semin. Cienc. Agrar. 2011, 32, 1893–1900. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical Partitioning and Antioxidant Capacity of Green Coffee (Coffea arabica and Coffea canephora) of Different Geographical Origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef]
- Mannino, G.; Kunz, R.; Maffei, M.E. Discrimination of Green Coffee (Coffea arabica and Coffea canephora) of Different Geographical Origin Based on Antioxidant Activity, High-Throughput Metabolomics, and DNA RFLP Fingerprinting. Antioxidants 2023, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Bloukas, J.G.; Paneras, E.D.; Fournitzis, G.C. Effect of Replacing Pork Backfat with Olive Oil on Processing and Quality Characteristics of Fermented Sausages. Meat Sci. 1997, 45, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, H.M.; Kumar, S.; Bhat, Z.F. Effect of Green Coffee Bean Extract on the Lipid Oxidative Stability and Storage Quality of Restructured Mutton Blocks Containing Colocasia Esculenta, a Novel Binding Agent. Agric. Res. 2017, 6, 443–454. [Google Scholar] [CrossRef]
- Jully, K.M.M.; Toto, C.S.; Were, L. Antioxidant Effect of Spent, Ground, and Lyophilized Brew from Roasted Coffee in Frozen Cooked Pork Patties. LWT—Food Sci. Technol. 2016, 66, 244–251. [Google Scholar] [CrossRef]
- Lin, C.; Toto, C.; Were, L. Antioxidant Effectiveness of Ground Roasted Coffee in Raw Ground Top Round Beef with Added Sodium Chloride. LWT—Food Sci. Technol. 2015, 60, 29–35. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic Acid-Mediated Gel Formation of Oxidatively Stressed Myofibrillar Protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Palmioli, A.; Airoldi, C. Coffee Variety, Origin and Extraction Procedure: Implications for Coffee Beneficial Effects on Human Health. Food Chem. 2019, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]



| Experiment No. | Extraction Conditions Real (Code) Levels 1 | Bioactive Compounds 2 | Antioxidant Activity 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | |
| (min) | (%) | g/mL | (AU) | (mg/ 100 g) | (mg/ 100 g) | (mg/ 100 g) | (g GAE/ 100 g) | (g TE/ 100 g) | |
| 1 | 5 (−1) | 25 (−1) | 5/100 (−1) | 0.331± 0.004 | 1401.46 ± 71.44 | 175.14 ± 13.03 | 679.24 ± 42.71 | 5.24 ± 0.09 | 11.82 ± 0.89 |
| 2 | 15 (1) | 25 (−1) | 5/100 (−1) | 0.352 ± 0.005 | 1379.94 ± 108.22 | 163.37 ± 13.55 | 681.08 ± 22.22 | 5.02 ± 0.15 | 11.14 ± 0.74 |
| 3 | 5 (−1) | 35 (1) | 5/100 (−1) | 0.748 ± 0.003 | 1368.91 ± 35.73 | 162.86 ± 8.18 | 607.23 ± 49.75 | 5.61 ± 0.12 | 11.06 ± 0.69 |
| 4 | 15 (1) | 35 (1) | 5/100 (−1) | 0.778 ± 0.005 | 1490.01 ± 157.07 | 190.16 ± 2.70 | 971.09 ± 45.58 | 6.28 ± 0.12 | 14.56 ± 0.62 |
| 5 | 5 (−1) | 25 (−1) | 15/100 (1) | 0.332 ± 0.004 | 1302.00 ± 117.03 | 160.13 ± 5.72 | 626.33 ± 44.17 | 4.39 ± 0.06 | 10.58 ± 0.17 |
| 6 | 15 (1) | 25 (−1) | 15/100 (1) | 0.321 ± 0.004 | 1281.87 ± 33.94 | 165.19 ± 7.36 | 633.38 ± 61.83 | 4.74 ± 0.05 | 13.78 ± 0.25 |
| 7 | 5 (−1) | 35 (1) | 15/100 (1) | 0.330 ± 0.004 | 1267.25 ± 29.55 | 169.94 ± 8.87 | 740.82 ± 59.29 | 4.73 ± 0.09 | 8.17 ± 0.25 |
| 8 | 15 (1) | 35 (1) | 15/100 (1) | 0.312 ± 0.003 | 1303.71 ± 19.19 | 182.53 ± 16.41 | 628.33 ± 27.80 | 4.90 ± 0.03 | 8.26 ± 0.22 |
| 9 | 1.6 (−1.68) | 30 (0) | 10/100 (0) | 0.503 ± 0.004 | 1298.55 ± 24.55 | 139.40 ± 1.91 | 625.51 ± 40.95 | 4.81 ± 0.05 | 8.81 ± 0.27 |
| 10 | 18.4 (1.68) | 30 (0) | 10/100 (0) | 0.506 ± 0.004 | 1442.32 ± 163.96 | 167.06 ± 2.08 | 699.77 ± 12.21 | 5.14 ± 0.07 | 9.70 ± 0.24 |
| 11 | 10 (0) | 21.6 (−1.68) | 10/100 (0) | 0.236 ± 0.003 | 1241.14 ± 28.10 | 137.90 ± 5.43 | 503.90 ± 33.28 | 4.14 ± 0.07 | 6.61 ± 0.39 |
| 12 | 10 (0) | 38.4 (1.68) | 10/100 (0) | 0.531 ± 0.004 | 1427.44 ± 144.76 | 165.75 ± 16.10 | 717.63 ± 75.48 | 5.39 ± 0.07 | 9.07 ± 0.27 |
| 13 | 10 (0) | 30 (0) | 1.6/100 (−1.68) | 0.473 ± 0.005 | 1362.71 ± 24.86 | 137.15 ± 5.82 | 688.36 ± 45.36 | 7.22 ± 0.31 | 21.37 ± 1.10 |
| 14 | 10 (0) | 30 (0) | 18.4/100 (1.68) | 0.290 ± 0.003 | 1254.17 ± 47.99 | 123.14 ± 2.84 | 647.21 ± 9.60 | 4.51 ± 0.04 | 7.96 ± 1.9 |
| 15 | 10 (0) | 30 (0) | 10/100 (0) | 0.408 ± 0.003 | 1485.43 ± 168.88 | 263.78 ± 7.89 | 1309.40 ± 14.45 | 4.72 ± 0.08 | 8.58 ± 0.13 |
| 16 | 10 (0) | 30 (0) | 10/100 (0) | 0.408 ± 0.004 | 1502.14 ± 92.59 | 269.66 ± 3.78 | 1254.85 ± 92.98 | 4.77 ± 0.08 | 8.16 ± 0.16 |
| 17 | 10 (0) | 30 (0) | 10/100 (0) | 0.406 ± 0.003 | 1358.34 ± 130.73 | 266.72 ± 5.84 | 1282.12 ± 71.60 | 4.79 ± 0.00 | 8.45 ± 0.23 |
| Parameter 1 | Effect | Standard Error | t (7) | p-Value | Effect | Standard Error | t (7) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Browned compounds | Total chlorogenic acids | |||||||
| Mean | 0.004 | 0.025 | 16.210 | 0.000 | 1276.202 | 53.313 | 23.938 | 0.000 |
| x1 | 0.075 | 0.024 | 0.180 | 0.862 | 56.434 | 50.102 | 1.126 | 0.297 |
| x2 | 0.194 | 0.026 | 2.894 | 0.023 | −399.337 | 55.196 | −7.235 | 0.000 |
| x3 | −0.011 | 0.024 | 8.256 | 0.000 | 100.625 | 50.102 | 2.008 | 0.085 |
| x12 | −0.179 | 0.026 | −0.421 | 0.686 | −436.096 | 55.196 | −7.901 | 0.000 |
| x22 | −0.012 | 0.024 | −7.606 | 0.000 | −55.538 | 50.102 | −1.108 | 0.304 |
| x32 | 0.001 | 0.026 | −0.468 | 0.654 | −395.688 | 55.196 | −7.169 | 0.000 |
| x1x2 | −0.020 | 0.031 | 0.020 | 0.985 | 60.621 | 65.433 | 0.926 | 0.385 |
| x1x3 | −0.214 | 0.031 | −0.650 | 0.536 | −117.783 | 65.433 | −1.800 | 0.115 |
| x2x3 | 0.004 | 0.031 | −6.943 | 0.000 | −27.139 | 65.433 | −0.415 | 0.691 |
| Caffeine | Antioxidant activity by Folin–Ciocalteau | |||||||
| Mean | 1447.391 | 35.876 | 40.344 | 0.000 | 4.765 | 0.183 | 26.078 | 0.000 |
| x1 | 52.396 | 33.715 | 1.554 | 0.164 | 0.222 | 0.172 | 1.294 | 0.237 |
| x2 | −47.090 | 37.143 | −1.268 | 0.245 | 0.101 | 0.189 | 0.534 | 0.610 |
| x3 | 55.350 | 33.715 | 1.642 | 0.145 | 0.620 | 0.172 | 3.610 | 0.009 |
| x12 | −72.704 | 37.143 | −1.957 | 0.091 | −0.048 | 0.189 | −0.255 | 0.806 |
| x22 | −97.890 | 33.715 | −2.903 | 0.023 | −1.163 | 0.172 | −6.773 | 0.000 |
| x32 | −91.023 | 37.143 | −2.451 | 0.044 | 0.734 | 0.189 | 3.879 | 0.006 |
| x1x2 | 49.801 | 44.031 | 1.131 | 0.295 | 0.182 | 0.224 | 0.811 | 0.444 |
| x1x3 | −20.811 | 44.031 | −0.473 | 0.651 | 0.017 | 0.224 | 0.078 | 0.940 |
| x2x3 | −22.605 | 44.031 | −0.513 | 0.623 | −0.284 | 0.224 | −1.265 | 0.246 |
| 5-caffeoilquinic acid | ABTS•+ scavenging activity | |||||||
| Mean | 264.471 | 13.546 | 19.525 | 0.000 | 8.367 | 1.456 | 5.747 | 0.001 |
| x1 | 11.674 | 12.730 | 0.917 | 0.390 | 1.118 | 1.368 | 0.817 | 0.441 |
| x2 | −65.357 | 14.024 | −4.660 | 0.002 | 0.808 | 1.507 | 0.536 | 0.609 |
| x3 | 12.965 | 12.730 | 1.018 | 0.342 | −0.165 | 1.368 | −0.121 | 0.907 |
| x12 | −66.357 | 14.024 | −4.732 | 0.002 | −0.196 | 1.507 | −0.130 | 0.900 |
| x22 | −5.464 | 12.730 | −0.429 | 0.681 | −4.442 | 1.368 | −3.247 | 0.014 |
| x32 | −81.717 | 14.024 | −5.827 | 0.001 | 4.641 | 1.507 | 3.079 | 0.018 |
| x1x2 | 11.645 | 16.625 | 0.700 | 0.506 | 0.268 | 1.787 | 0.150 | 0.885 |
| x1x3 | 0.529 | 16.625 | 0.032 | 0.976 | 0.118 | 1.787 | 0.066 | 0.949 |
| x2x3 | 3.162 | 16.625 | 0.190 | 0.855 | −2.648 | 1.787 | −1.482 | 0.182 |
| Parameter | Factor Variable | Sum of Squares | Degree of Freedom | Mean Square | F | Fcritical | p-Value |
|---|---|---|---|---|---|---|---|
| Browned compounds | Regression | 0.351 | 5 | 0.070 | 52.746 | 3.204 | 0.000 |
| Residue | 0.015 | 11 | 0.001 | ||||
| Total | 0.366 | 16 | 0.023 | ||||
| Caffeine | Regression | 87,551.36 | 7 | 12,507.34 | 3.877 | 3.293 | 0.031 |
| Residue | 29,030.81 | 9 | 3225.65 | ||||
| Total | 116,582 | 16 | 7286.39 | ||||
| Acid 5-caffeoylquinic | Regression | 28,944.07 | 7 | 4134.87 | 9.567 | 3.293 | 0.002 |
| Residue | 3889.89 | 9 | 432.21 | ||||
| Total | 32,833.96 | 16 | 2052.12 | ||||
| Total chlorogenic acids | Regression | 981,445.44 | 7 | 140,206.49 | 18.351 | 3.293 | 0.000 |
| Residue | 68,763.91 | 9 | 7640.32 | ||||
| Total | 1,050,208.35 | 16 | 65,638.02 | ||||
| Antioxidant activity (Folin–Ciocalteau) | Regression | 7.94516 | 5 | 1.589 | 21.357 | 3.204 | 0.000 |
| Residue | 0.818425 | 11 | 0.074 | ||||
| Total | 8.763587 | 16 | 0.548 | ||||
| ABTS•+ scavenging activity | Regression | 151.45410 | 5 | 30.291 | 7.047 | 3.204 | 0.004 |
| Residue | 47.2831 | 11 | 4.298 | ||||
| Total | 198.7372 | 16 | 12.421 |
| Sample 1 | Browned Compounds (UA) 2 | Caffeine (mg/100 g) 2 | 5-Caffeoylquinic (mg/100 g) 2 | Folin–Ciocalteau (g EAG/100 g) 2 | ABTS+• (g Eq Trolox/100 g) 2 |
|---|---|---|---|---|---|
| ORR | 0.364 ± 0.013 b | 1881.8 ± 18.8 c | 482.9 ± 21.6 c | 8.66 ± 0.14 b | 16.28 ± 1.00 b |
| OAR | 0.505 ± 0.035 a | 1652.6 ± 47.4 d | 703.8 ± 38.3 c | 4.29 ± 0.19 g | 12.09 ± 0.38 d |
| CRR | 0.303 ± 0.016 c | 1764.1 ± 34,8 d | 427.3 ± 8.1 c | 7.36 ± 0.32 d | 15.28 ± 1.47 bc |
| CAR | 0.379 ± 0.018 b | 1244.9 ± 52.0 e | 464.5 ± 35.7 c | 3.41 ± 0.14 h | 11.61 ± 1.22 d |
| ORG | - | 3148.1 ± 13.5 a | 6706.4 ± 23.5 a | 9.54 ± 0.40 a | 14.52 ± 1.16 c |
| OAG | - | 2145.0 ± 106.9 b | 5439.6 ± 393.1 b | 6.35 ± 0.20 e | 20.54 ± 1.04 a |
| CRG | - | 3114.8 ± 50.0 a | 6417.1 ± 22.0 a | 8.04 ± 0.14 c | 12.93 ± 0.78 d |
| CAG | - | 2210.8 ± 43.9 b | 5286.8 ± 259.5 b | 5.72 ± 0.17 f | 15.31 ± 0.34 bc |
| Antioxidant | |||||
| DORR | - | - | - | 27.25 ± 0.16 | 41.35 ± 1.12 |
| DORG | - | - | - | 24.79 ± 0.84 | 42.93 ± 1.57 |
| SE | - | - | - | 80.01 ± 0.46 | 124.70 ± 0.92 |
| Sample 1 | TBARs (mg of Malonaldehyde/kg of Sample) (n = 3) | Sensory Acceptance 2 (n = 62) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 10 Day | 20 Day | Smell 3 | Color 3 | Flavor 3 | Texture 3 | Global Acceptance 3 | Purchase Intention 4 | ||
| GDE1% | 0.681 ± 0.056 Aa | 0.696 ± 0.059 Aa | 0.677 ± 0.068 Aa | 6.76 ± 1.50 c | 7.19 ± 1.39 b | 7.16 ± 1.43 bc | 7.32 ± 1.56 a | 7.34 ± 1.09 bc | 3.85 ± 0.91 bc | |
| GDE032% | 0.612 ± 0.056 Aa | 0.595 ± 0.071 Aa | 0.577 ± 0.079 Aa | 7.43 ± 1.16 ab | 7.71 ± 1.05 ab | 7.63 ± 1.37 ab | 7.68 ± 1.19 a | 7.77 ± 1.07 ab | 4.21 ± 0.86 ab | |
| C | 0.662 ± 0.038 Aa | 0.697 ± 0.065 Aa | 0.628 ± 0.048 Aa | 7.81 ± 1.24 a | 8.00 ± 0.88 a | 8.19 ± 1.01 a | 7.92 ± 1.37 a | 8.19 ± 0.91 a | 4.53 ± 0.98 a | |
| RDE032% | 0.671 ± 0.056 Aa | 0.671 ± 0.049 Aa | 0.687 ± 0.067 Aa | 6.84 ± 1.73 bc | 6.15 ± 1.94 c | 6.92 ± 1.85 c | 7.31 ± 1.56 a | 7.03 ± 1.48 c | 3.66 ± 1.07 c | |
| RDE1% | 0.655 ± 0.054 Ab | 0.687 ± 0.045 Ab | 0.644 ± 0.071 Ab | 6.84 ± 1.73 bc | 6.84 ± 1.73 bc | 6.84 ± 1.73 bc | 6.84 ± 1.73 bc | 6.84 ± 1.73 bc | 6.84 ± 1.73 bc | |
| Texture parameters (n = 3) 2 | Color parameters of raw sausages (n = 3) 2 | Color parameters of bakedsausages (n = 3) 2 | ||||||||
| Hardness(N) | Elasticity(mm) | Chewiness(N/mm) | Cohesiveness | L* | a* | b* | L* | a* | b* | |
| GDE1% | 44.55 ± 3.37 a | 0.843 ± 0.008 a | 23.79 ± 3.06 a | 0.551 ± 0.048 c | 55.07 ± 2.92 ab | 14.85 ± 0.90 a | 14.77 ± 0.07 b | 60.58 ± 0.90 a | 9.91 ± 0.96 b | 14.07 ± 0.70 b |
| GDE032% | 47.79 ± 4.23 a | 0.854 ± 0.020 a | 25.23 ± 0.56 a | 0.505 ± 0.005 c | 59.54 ± 0.87 a | 8.59 ± 1.64 c | 11.66 ± 0.30 c | 60.63 ± 1.23 a | 13.04 ± 0.27 a | 11.19 ± 0.71 c |
| C | 42.38 ± 0.87 a | 0.892 ± 0.003 a | 22.35 ± 1.02 a | 0.581 ± 0.007 bc | 58.06 ± 1.83 a | 14.49 ± 0.90 a | 10.87 ± 0.69 c | 60.99 ± 1.33 a | 14.87 ± 0.41 a | 10.64 ± 0.30 c |
| RDE032% | 47.37 ± 1.24 a | 0.826 ± 0.037 a | 25.58 ± 1.04 a | 0.663 ± 0.039 ab | 51.91 ± 1.02 b | 12.58 ± 0.46 ab | 17.96 ± 0.62 a | 49.31 ± 2.60 b | 9.97 ± 0.75 b | 17.67 ± 0.20 a |
| RDE1% | 46.63 ± 1.28 a | 0.843 ± 0.027 a | 23.74 ± 0.30 a | 0.694 ± 0.036 a | 40.58 ± 1.37 c | 11.67 ± 0.50 b | 16.74 ± 1.22 ab | 40.31 ± 0.68 c | 9.33 ± 0.33 b | 14.3 ± 0.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetsch, V.T.; Kalschne, D.L.; Canan, C.; Flores, É.L.d.M.; Viegas, M.C.; Peiter, G.C.; Zara, R.F.; Amaral, J.S.; Corso, M.P. Coffee Extract as a Natural Antioxidant in Fresh Pork Sausage—A Model Approach. Foods 2024, 13, 1409. https://doi.org/10.3390/foods13091409
Fetsch VT, Kalschne DL, Canan C, Flores ÉLdM, Viegas MC, Peiter GC, Zara RF, Amaral JS, Corso MP. Coffee Extract as a Natural Antioxidant in Fresh Pork Sausage—A Model Approach. Foods. 2024; 13(9):1409. https://doi.org/10.3390/foods13091409
Chicago/Turabian StyleFetsch, Vanessa Tanara, Daneysa Lahis Kalschne, Cristiane Canan, Éder Lisandro de Moraes Flores, Marcelo Caldeira Viegas, Gabrielle Caroline Peiter, Ricardo Fiori Zara, Joana Soares Amaral, and Marinês Paula Corso. 2024. "Coffee Extract as a Natural Antioxidant in Fresh Pork Sausage—A Model Approach" Foods 13, no. 9: 1409. https://doi.org/10.3390/foods13091409