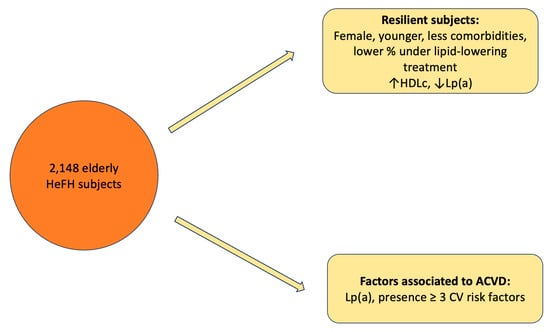
Resilient Older Subjects with Heterozygous Familial Hypercholesterolemia, Baseline Differences and Associated Factors
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Current Lipid Profile
2.3. Factors Associated with the Presence of ACVD
3. Discussion
4. Materials and Methods
4.1. Study Characteristics
4.2. Study Variables
4.3. SEA Dyslipidemia Registry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, M.S.; Goldstein, J.L. Familial hypercholesterolemia: A genetic defect in the low-density lipoprotein receptor. N. Engl. J. Med. 1976, 294, 1386–1390. [Google Scholar] [PubMed]
- Nnerarity, T.; Mahley, R.; Weisgraber, K.; Bersot, T.; Krauss, R.; Vega, G.; Grundy, S.; Friedl, W.; Davignon, J.; McCarthy, B. Familial defective apolipoprotein B-100: A mutation of apolipoprotein B that causes hypercholesterolemia. J. Lipid Res. 1990, 31, 1337–1349. [Google Scholar] [CrossRef]
- Abifadel, M.; Rabès, J.-P.; Devillers, M.; Munnich, A.; Erlich, D.; Junien, C.; Varret, M.; Boileau, C. Mutations and polymorphisms in the proprotein convertase subtilisin/kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 2009, 30, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Cenarro, A.; Etxebarria, A.; de Castro-Orós, I.; Stef, M.; Bea, A.M.; Palacios, L.; Mateo-Gallego, R.; Benito-Vicente, A.; Ostolaza, H.; Tejedor, T.; et al. The p. Leu167del mutation in APOE gene causes autosomal dominant hypercholesterolemia by down-regulation of LDL receptor expression in hepatocytes. J. Clin. Endocrinol. Metab. 2016, 101, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 2017, 3, 17093. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H. High risk/high priority: Familial hypercholesterolemia—A paradigm for molecular medicine. Atheroscler. Suppl. 2002, 2, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sijbrands, E.J.; Kaprio, J.; Westendorp, R.G.; Defesche, J.C.; de Meier, P.H.; Smelt, A.H.; Kastelein, J.J. Mortality over two centuries in large pedigree with familial hypercholesterolaemia: Family tree mortality study. BMJ 2001, 322, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Mata, N.; Castillo, S.; Fuentes, F.; Saenz, P.; Muñiz, O.; Galiana, J.; Figueras, R.; Diaz, J.; Gomez-Enterría, P.; et al. Cardiovascular disease in familial hypercholesterolaemia: Influence of low-density lipoprotein receptor mutation type and classic risk factors. Atherosclerosis 2008, 200, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.R.; Miname, M.H.; Rocha, V.Z.; Bittencourt, M.S.; Jannes, C.E.; Tada, M.T.; Lima, I.R.; Filho, W.S.; Chacra, A.P.; Pereira, A.C.; et al. Familial hypercholesterolemia and cardiovascular disease in older individuals. Atherosclerosis 2021, 318, 32–37. [Google Scholar] [CrossRef] [PubMed]
- EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- De Isla, L.P.; Watts, G.F.; Muñiz-Grijalvo, O.; Díaz-Díaz, J.L.; Alonso, R.; Zambón, D.; Fuentes-Jimenez, F.; Mauri, M.; Padró, T.; I Vidal-Pardo, J.; et al. A resilient type of familial hypercholesterolaemia: Case-control follow-up of genetically characterized older patients in the SAFEHEART cohort. Eur. J. Prev. Cardiol. 2022, 29, 795–801. [Google Scholar] [CrossRef]
- Benn, M.; Watts, G.F.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Familial hypercholesterolemia in the Danish general population: Prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 2012, 97, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- De Sauvage Nolting, P.R.W.; Defesche, J.C.; Buirma, R.J.A.; Hutten, B.A.; Lansberg, P.J.; Kastelein, J.J.P. Prevalence and significance of cardiovascular risk factors in a large cohort of patients with familial hypercholesterolaemia. J. Intern. Med. 2003, 253, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Real, J.; Chaves, F.; Martínez-Usó, I.; García-García, A.; Ascaso, J.; Carmena, R. Importance of HDL cholesterol levels and the total/HDL cholesterol ratio as a risk factor for coronary heart disease in molecularly defined heterozygous familial hypercholesterolaemia. Eur. Heart J. 2001, 22, 465–471. [Google Scholar] [CrossRef]
- Khoury, E.; Brisson, D.; Roy, N.; Tremblay, G.; Gaudet, D. Identifying markers of cardiovascular event-free survival in familial hypercholesterolemia. J. Clin. Med. 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Caggiula, A.W.; Wing, R.R. Menopause and risk factors for coronary heart disease. N. Engl. J. Med. 1989, 321, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Perak, A.M.; Ning, H.; De Ferranti, S.D.; Gooding, H.C.; Wilkins, J.T.; Lloyd-Jones, D.M. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation 2016, 134, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Banach, M.; Pirro, M.; International Lipid Expert Panel (ILEP). Why patients with familial hypercholesterolemia are at high cardiovascular risk? Beyond LDL-C levels. Trends Cardiovasc. Med. 2021, 31, 205–215. [Google Scholar] [CrossRef] [PubMed]
- De Isla, L.P.; Alonso, R.; Argüeso, R.; Muñiz-Grijalvo, O.; Álvarez-Baños, P.; Badimón, L.; Watts, G.F.; Mata, P.; SAFEHEART Investigators. Predicting resilience in heterozygous familial hypercholesterolaemia: A cohort study of octogenarian patients. J. Clin. Lipidol. 2022, 16, 733–736. [Google Scholar] [CrossRef]
- Melnes, T.; Bogsrud, M.P.; Thorsen, I.; Fossum, J.; Christensen, J.J.; Narverud, I.; Retterstøl, K.; Ulven, S.M.; Holven, K.B. What characterizes event-free elderly FH patients? A comprehensive lipoprotein profiling. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Familial hypercholesterolemia: Do HDL play a role? Biomedicines 2021, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Tall, A.R. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat. Med. 2012, 18, 1344–1346. [Google Scholar] [CrossRef]
- Cao, Y.-X.; Jin, J.-L.; Guo, Y.-L.; Sun, D.; Liu, H.-H.; Wu, N.-Q.; Xu, R.-X.; Zhu, C.-G.; Liu, G.; Dong, Q.; et al. Baseline and on-statin treatment lipoprotein(a) levels for predicting cardiovascular events in patients with familial hypercholesterolemia. Atherosclerosis 2019, 291, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, N.-Q.; Zhu, C.-G.; Zhang, Y.; Guo, Y.-L.; Gao, Y.; Li, X.-L.; Qing, P.; Cui, C.-J.; Xu, R.-X.; et al. Significance of lipoprotein(a) levels in familial hypercholesterolemia and coronary artery disease. Atherosclerosis 2017, 260, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-C.; Chapman, J.; Bruckert, E.; Farriaux, J.-P.; De Gennes, J.-L. Lipoprotein Lp(a) in homozygous familial hypercholesterolemia: Density profile, particle heterogeneity and apolipoprotein(a) phenotype. Atherosclerosis 1991, 86, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, G.; Cadeddu Dessalvi, C.; Deidda, M. To be or not to be resilient in familial hypercholesterolaemia: Implications for the management. Eur. J. Prev. Cardiol. 2022, 29, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.; Hamkour, S.; Siegers, K.; Holven, K.; Johansen, A.; van de Ree, M.; Imholz, B.; Boersma, E.; Louters, L.; Bogsrud, M.; et al. LDL cholesterol targets rarely achieved in familial hypercholesterolemia patients: A sex and gender-specific analysis. Atherosclerosis 2023, 384, 117117. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Calahorra, S.; Sánchez-Hernández, R.M.; Plana, N.; Valdivielso, P.; Civeira, F. National dyslipidemia registry of the Spanish Arteriosclerosis Society: Current status. Clin. Investig. Arterioscler. 2017, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
| Variable | Non-ACVD | ACVD | p |
|---|---|---|---|
| Total, n | 1583 | 565 | |
| Male sex, n (%) | 586 (37.0) | 389 (68.8) | <0.001 |
| Age (years), mean ± SD | 76.6 ± 5.9 | 77.9 ± 6.6 | <0.001 |
| BMI (kg/m2), mean ± SD | 27.8 ± 4.2 | 28.8 ± 4.3 | <0.001 |
| Family History | |||
| Paternal ACVD, n (%) | 254 (16.0) | 91 (16.1) | <0.001 |
| Maternal ACVD, n (%) | 158 (10.0) | 56 (9.9) | <0.001 |
| Premature ACVD in a first-degree relative, n (%) | 312 (19.7) | 143 (25.3) | <0.001 |
| Personal History | |||
| T2DM, n (%) | 400 (25.3) | 238 (42.1) | <0.001 |
| Hypertension, n (%) | 704 (44.5) | 315 (53.8) | <0.001 |
| Systolic blood pressure (mmHg), mean ± SD | 132.7 ± 27.6 | 130.2 ± 53.7 | 0.138 |
| Diastolic blood pressure (mmHg), mean ± SD | 78.5 ± 23.7 | 73.8 ± 44.7 | 0.009 |
| Tendon xanthoma, n (%) | 157 (9.9) | 66 (11.7) | <0.001 |
| Corneal arch, n (%) | 365 (23.1) | 131 (23.2) | <0.001 |
| Current smokers, n (%) | 193 (12.2) | 55 (9.7) | <0.001 |
| Positive genetic mutation, n (%) | 287 (18.1) | 132 (23.4) | <0.001 |
| Type genetic mutation, n (%) | LDLR 267 (93.0) APOB 18 (6.3) PCSK9 2 (0.7) APOE 0 (0) | LDLR 121 (91.7) APOB 10 (7.5) PCSK9 0 (0) APOE 1 (0.8) | 0.342 |
| Variable | Non-ACVD | ACVD | p |
|---|---|---|---|
| Total cholesterol (mg/dL), mean ± SD | 299.4 ± 89.6 | 284.9 ± 112.8 | 0.003 |
| HDL cholesterol (mg/dL), mean ± SD | 55.8 ± 17.1 | 47.9 ± 15.4 | <0.001 |
| Non-HDL cholesterol (mg/dL), mean ± SD | 243.6 ± 87.2 | 237.0 ± 109.7 | 0.099 |
| LDL cholesterol (mg/dL), mean ± SD | 194.4 ± 98.7 | 187.8 ± 115.0 | 0.112 |
| Triglycerides (mg/dL), mean ± SD | 208.3 ± 378.5 | 206.9 ± 227.3 | 0.467 |
| Lp(a) (mg/dL), mean ± SD | 53.4 ± 67.9 | 66.6 ± 85.6 | <0.001 |
| Variable | Non-ACVD | ACVD | p |
|---|---|---|---|
| Total cholesterol (mg/dL), mean ± SD | 198.9 ± 52.2 | 172.0 ± 51.3 | <0.001 |
| HDL cholesterol (mg/dL), mean ± SD | 56.7 ± 16.0 | 48.6 ± 14.7 | <0.001 |
| Non-HDL cholesterol (mg/dL), mean ± SD | 142.0 ± 48.6 | 123.4 ± 48.6 | <0.001 |
| LDL cholesterol (mg/dL), mean ± SD | 126.3 ± 48.4 | 96.0 ± 49.3 | <0.001 |
| Triglycerides (mg/dL), mean ± SD | 144.2 ± 152.8 | 160.1 ± 148.1 | <0.001 |
| OR (95% CI) | p | |
|---|---|---|
| Male sex | 1.698 (0.768–3.758) | 0.191 |
| Age | 1.053 (0.988–1.122) | 0.115 |
| BMI, kg/m2 | 0.995 (0.975–1.015) | 0.604 |
| T2DM | 1.058 (0.456–2.455) | 0.896 |
| Hypertension | 0.909 (0.410–2.017) | 0.815 |
| Active smoking | 1.833 (0.403–8.340) | 0.433 |
| Total cholesterol (mg/dL) | 0.962 (0.921–1.006) | 0.087 |
| HDL cholesterol (mg/dL) | 1.035 (0.958–1.119) | 0.383 |
| LDL cholesterol (mg/dL) | 1.030 (0.991–1.070) | 0.130 |
| Triglycerides (mg/dL) | 1.012 (1.000–1.024) | 0.050 |
| Lp(a) (mg/dL) | 1.002 (1.000–1.004) | 0.019 |
| Positive genetic mutation | 1.231 (0.659–2.297) | 0.515 |
| Presence ≥ 3 CV risk factors | 2.795 (1.950–4.005) | <0.001 |
| Statin therapy > 30 years or initiation < 40 years of age | 0.442 (0.024–8.306) | 0.586 |
| Lp(a) > 50 mg/dL | 1.183 (0.099–14.172) | 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent, E.; González-Guerrero, A.; Marco-Benedí, V.; García-Andreu, M.-d.-M.; Mediavilla-García, J.D.; Suárez-Tembra, M.; Benaiges, D.; Pintó, X.; Pedro-Botet, J. Resilient Older Subjects with Heterozygous Familial Hypercholesterolemia, Baseline Differences and Associated Factors. Int. J. Mol. Sci. 2024, 25, 4831. https://doi.org/10.3390/ijms25094831
Climent E, González-Guerrero A, Marco-Benedí V, García-Andreu M-d-M, Mediavilla-García JD, Suárez-Tembra M, Benaiges D, Pintó X, Pedro-Botet J. Resilient Older Subjects with Heterozygous Familial Hypercholesterolemia, Baseline Differences and Associated Factors. International Journal of Molecular Sciences. 2024; 25(9):4831. https://doi.org/10.3390/ijms25094831
Chicago/Turabian StyleCliment, Elisenda, Antón González-Guerrero, Victoria Marco-Benedí, María-del-Mar García-Andreu, Juan Diego Mediavilla-García, Manuel Suárez-Tembra, David Benaiges, Xavier Pintó, and Juan Pedro-Botet. 2024. "Resilient Older Subjects with Heterozygous Familial Hypercholesterolemia, Baseline Differences and Associated Factors" International Journal of Molecular Sciences 25, no. 9: 4831. https://doi.org/10.3390/ijms25094831