Adaptive Fabrication of Electrochemical Chips with a Paste-Dispensing 3D Printer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
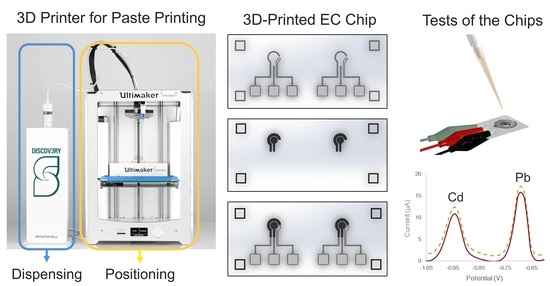
2.2. Preparations for 3D Printing
2.3. EC Chip Fabrication
2.4. Performance Tests
3. Results and Discussion
3.1. Fabrication Process Optimization
3.2. Thicknesses of the 3D-Printed Electrodes
3.3. Insulating Layer
3.4. Cyclic Voltammetry of Fabricated EC Chip
3.5. Differential Pulse Anodic Stripping Voltammetry with Standard Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPASV | differential pulse anodic stripping voltammetry |
| CV | cyclic voltammetry |
| EC | electrochemical |
| PMMA | poly(methyl methacrylate) |
| PTFE | polytetrafluoroethylene |
References
- Wang, J. Stripping Analysis: Principles, Instrumentation, and Applications; Vch Pub: New York, NY, USA, 1985. [Google Scholar]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702. [Google Scholar] [CrossRef]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7, 616787. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, B.; Nascimento, V.B.; Angnes, L. Performance of screen-printed carbon electrodes fabricated from different carbon inks. Electrochim. Acta 1998, 43, 3459–3465. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, P.; McGaw, E.; Show, Y.; Swain, G.M. Metal ion analysis in contaminated water samples using anodic stripping voltammetry and a nanocrystalline diamond thin-film electrode. Anal. Chim. Acta 2004, 522, 35–44. [Google Scholar] [CrossRef]
- Palchetti, I.; Laschi, S.; Mascini, M. Miniaturised stripping-based carbon modified sensor for in field analysis of heavy metals. Anal. Chim. Acta 2005, 530, 61–67. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Disposable Bismuth Oxide Screen Printed Electrodes for the High Throughput Screening of Heavy Metals. Electroanalysis 2009, 21, 2410–2414. [Google Scholar] [CrossRef]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Enhanced electrochemical detection of heavy metals at heated graphite nanoparticle-based screen-printed electrodes. J. Mater. Chem. 2011, 21, 4326–4331. [Google Scholar] [CrossRef]
- Silva-Neto, H.A.; Dias, A.A.; Coltro, W.K.T. 3D-printed electrochemical platform with multi-purpose carbon black sensing electrodes. Microchim. Acta 2022, 189, 235. [Google Scholar] [CrossRef] [PubMed]
- Baltima, A.; Panagopoulou, H.; Economou, A.; Kokkinos, C. 3D-printed fluidic electrochemical microcell for sequential injection/stripping analysis of heavy metals. Anal. Chim. Acta 2021, 1159, 338426. [Google Scholar] [CrossRef]
- Stefano, J.S.; Kalinke, C.; da Rocha, R.G.; Rocha, D.P.; da Silva, V.A.O.P.; Bonacin, J.A.; Angnes, L.; Richter, E.M.; Janegitz, B.C.; Muñoz, R.A.A. Electrochemical (Bio)Sensors Enabled by Fused Deposition Modeling-Based 3D Printing: A Guide to Selecting Designs, Printing Parameters, and Post-Treatment Protocols. Anal. Chem. 2022, 94, 6417–6429. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, G.D. Toward single-step production of functional electrochemical devices using 3D printing: Progress, challenges, and opportunities. Curr. Opin. Electrochem. 2020, 20, 60–65. [Google Scholar] [CrossRef]
- Calderilla, C.; Maya, F.; Cerdà, V.; Leal, L.O. 3D printed device for the automated preconcentration and determination of chromium (VI). Talanta 2018, 184, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cao, Q.; Venton, B. 3D printing for customized carbon electrodes. Curr. Opin. Electrochem. 2023, 38, 101228. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Zaman, S.; Dantzler, J.Z.R.; Leyva, D.H.; Mahmud, M.S.; Ramirez, J.M.; Gomez, S.G.; Lin, Y. 3D Printed Integrated Sensors: From Fabrication to Applications—A Review. Nanomaterials 2023, 13, 3148. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Albuquerque, R.B.; Oliveira, G.P.; Cardoso, R.M.; Semaan, F.S.; Dornellas, R.M.; Richter, E.M.; Muñoz, R.A.A. Sensing Materials: Electrochemical Sensors Enabled by 3D Printing. In Encyclopedia of Sensors and Biosensors, 1st ed.; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 73–88. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Rocha, D.P.; Rocha, R.G.; Stefano, J.S.; Silva, R.A.; Richter, E.M.; noz, R.A.M. 3D-printing pen versus desktop 3D-printers: Fabrication of carbon black/polylactic acid electrodes for single-drop detection of 2,4,6-trinitrotoluene. Anal. Chim. Acta 2020, 1132, 10–19. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef]
- Carrasco-Correa, E.J.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Miró, M. The emerging role of 3D printing in the fabrication of detection systems. TrAC Trends Anal. Chem. 2021, 136, 116177. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Crapnell, R.D.; Rothwell, E.J.; Hurst, N.J.; Banks, C.E. Additive manufacturing for electrochemical labs: An overview and tutorial note on the production of cells, electrodes and accessories. Talanta Open 2021, 4, 100051. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ambrosi, A.; Pumera, M. 3D-printed Metal Electrodes for Heavy Metals Detection by Anodic Stripping Voltammetry. Electroanalysis 2017, 29, 2444–2453. [Google Scholar] [CrossRef]
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2016, 26, 698–703. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhao, Z.; Sun, W.; Zhao, G.; Liu, J.; Xu, J.; Li, Y.; Liu, Z.; Li, Y.; et al. Calotropis gigantea Fiber-Based Sensitivity-Tunable Strain Sensors with Insensitive Response to Wearable Microclimate Changes. Adv. Fiber Mater. 2023, 5, 1378–1391. [Google Scholar] [CrossRef]
- Ma, C.; Wang, M.; Wang, K.; Uzabakiriho, P.C.; Chen, X.; Zhao, G. Ultrasensitive, Highly Selective, Integrated Multidimensional Sensor Based on a Rigid-Flexible Synergistic Stretchable Substrate. Adv. Fiber Mater. 2023, 5, 1392–1403. [Google Scholar] [CrossRef]
- Xu, S.; Nie, W.; Sun, J.; Sun, P.; Jia, H.; Zheng, X.; Sun, Y.; Xu, Z.; Liu, L. Multi-mode and durable fiber triboelectric nanogenerator for power and sensor enabled by Hookean vascular stent structure. Chem. Eng. J. 2023, 472, 145088. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable Screen Printed Electrochemical Sensors: Tools for Environmental Monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.; Bown, E.; Watson, A.; Holman, R.; Steemson, J.; Hughes, S.; Scott, D. Pen-sized digital 30-second blood glucose meter. Lancet 1987, 329, 778–779. [Google Scholar] [CrossRef]
- Wang, J.; Pedrero, M.; Sakslund, H.; Hammerich, O.; Pingarron, J. Electrochemical activation of screen-printed carbon strips. Analyst 1996, 121, 345–350. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Laschi, S.; Palchetti, I.; Mascini, M. Gold-based screen-printed sensor for detection of trace lead. Sens. Actuators B Chem. 2006, 114, 460–465. [Google Scholar] [CrossRef]
- Noh, M.F.M.; Tothill, I.E. Development and characterisation of disposable gold electrodes, and their use for lead(II) analysis. Anal. Bioanal. Chem. 2006, 386, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Jothimuthu, P.; Wilson, R.A.; Herren, J.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Lab-on-a-chip sensor for detection of highly electronegative heavy metals by anodic stripping voltammetry. Biomed. Microdevices 2011, 13, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Masawat, P.; Liawruangrath, S.; Slater, J. Flow injection measurement of lead using mercury-free disposable gold-sputtered screen-printed carbon electrodes (SPCE). Sens. Actuators B Chem. 2003, 91, 52–59. [Google Scholar] [CrossRef]
- Kim, M.; Bon Koo, J.; Baeg, K.J.; Jung, S.W.; Ju, B.K.; You, I.K. Top-gate staggered poly(3,3′′′-dialkyl-quarterthiophene) organic thin-film transistors with reverse-offset-printed silver source/drain electrodes. Appl. Phys. Lett. 2012, 101, 133306. [Google Scholar] [CrossRef]
- Kang, H.; Park, H.; Park, Y.; Jung, M.; Kim, B.C.; Wallace, G.; Cho, G. Fully Roll-to-Roll Gravure Printable Wireless (13.56 MHz) Sensor-Signage Tags for Smart Packaging. Sci. Rep. 2014, 4, 5387. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H.; Kawase, T.; Friend, R.H.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E.P. High-Resolution Inkjet Printing of All-Polymer Transistor Circuits. Science 2000, 290, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Noguchi, Y.; Zschieschang, U.; Klauk, H.; Someya, T. Organic transistors manufactured using inkjet technology with subfemtoliter accuracy. Proc. Natl. Acad. Sci. USA 2008, 105, 4976–4980. [Google Scholar] [CrossRef]
- Shin, D.Y.; Grassia, P.; Derby, B. Numerical and experimental comparisons of mass transport rate in a piezoelectric drop-on-demand inkjet print head. Int. J. Mech. Sci. 2004, 46, 181–199. [Google Scholar] [CrossRef]
- Park, J.U.; Hardy, M.; Kang, S.J.; Barton, K.; Adair, K.; Mukhopadhyay, D.k.; Lee, C.Y.; Strano, M.S.; Alleyne, A.G.; Georgiadis, J.G.; et al. High-resolution electrohydrodynamic jet printing. Nat. Mater. 2007, 6, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, Y.; Ding, Y.; Bu, N.; Pan, Y.; Lu, N.; Yin, Z. Versatile, kinetically controlled, high precision electrohydrodynamic writing of micro/nanofibers. Sci. Rep. 2014, 4, 5949. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Seo, J.Y.; Tak, H.; Byun, D. Bimodally dispersed silver paste for the metallization of a crystalline silicon solar cell using electrohydrodynamic jet printing. Sol. Energy Mater. Sol. Cells 2015, 136, 148–156. [Google Scholar] [CrossRef]
- Mahajan, A.; Frisbie, C.D.; Francis, L.F. Optimization of Aerosol Jet Printing for High-Resolution, High-Aspect Ratio Silver Lines. ACS Appl. Mater. Interfaces 2013, 5, 4856–4864. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Yoo, S.S.; Song, H.e.; Tak, H.; Byun, D. Electrostatic-Force-Assisted Dispensing Printing to Construct High-Aspect-Ratio of 0.79 Electrodes on a Textured Surface with Improved Adhesion and Contact Resistivity. Sci. Rep. 2015, 5, 16704. [Google Scholar] [CrossRef] [PubMed]
- SunSens Electronic Materials for Medical Sensors. 2020. Available online: https://juhl-as.com/wp-content/uploads/2023/03/EM-Medical-Sensors-Biosens-BRO-EM002_RR_111720.pdf (accessed on 19 April 2024).
- Product Information: ARcare® 92712. 2010. Available online: https://parafix.com/wp-content/uploads/2019/01/adhesives-research-92712-tape-data-sheet-parafix.pdf (accessed on 19 April 2024).
- Boehm, F.H.J.; Melnick, B.D. Dual Chamber Syringe and Dual Lumen Needle. U.S. Patent 6,972,005 B2, 6 December 2005. Available online: https://patents.google.com/patent/US6972005B2 (accessed on 19 April 2024).
- Micrux SPE with Single Working Electrode. 2023. Available online: https://www.palmsens.com/product/micrux-thick-film-electrodes/ (accessed on 19 April 2024).
- Zensor Screen Printed Electrodes. 2023. Available online: https://www.nanoshel.com/product/zensor-screen-printed-electrodes (accessed on 19 April 2024).
- Ganguly, P.; Neethipathi, D.K.; Beniwal, A.; Dahiya, R. Influence of Thickness of Screen Printed Carbon Electrodes on Electrochemical Sensing. In Proceedings of the 2022 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Vienna, Austria, 10–13 July 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Bonaldo, S.; Franchin, L.; Rosati, G.; Tonello, S.; Merkoçi, A.; Paccagnella, A. Multiphysics simulations of screen-printed electrodes for electrochemical biosensing. In Proceedings of the 2023 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4.0&IoT), Brescia, Italy, 6–8 June 2023; pp. 320–325. [Google Scholar] [CrossRef]
- Obaje, E.A.; Cummins, G.; Schulze, H.; Mahmood, S.; Desmulliez, M.P.; Bachmann, T.T. Carbon screen-printed electrodes on ceramic substrates for label-free molecular detection of antibiotic resistance. J. Interdiscip. Nanomed. 2016, 1, 93–109. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]





| Material | PMMA (Substrate) | Ag/AgCl (First Layer) | Carbon (Second Layer) |
|---|---|---|---|
| Mean Values (mm) | 1.00 | 0.055 | 0.035 |
| Standard Deviation (mm) | 0.0063 | 0.023 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, T.I.; Ng, C.; Lin, S.; Chen, Z.; Zhou, X. Adaptive Fabrication of Electrochemical Chips with a Paste-Dispensing 3D Printer. Sensors 2024, 24, 2844. https://doi.org/10.3390/s24092844
Wong TI, Ng C, Lin S, Chen Z, Zhou X. Adaptive Fabrication of Electrochemical Chips with a Paste-Dispensing 3D Printer. Sensors. 2024; 24(9):2844. https://doi.org/10.3390/s24092844
Chicago/Turabian StyleWong, Ten It, Candy Ng, Shengxuan Lin, Zhong Chen, and Xiaodong Zhou. 2024. "Adaptive Fabrication of Electrochemical Chips with a Paste-Dispensing 3D Printer" Sensors 24, no. 9: 2844. https://doi.org/10.3390/s24092844