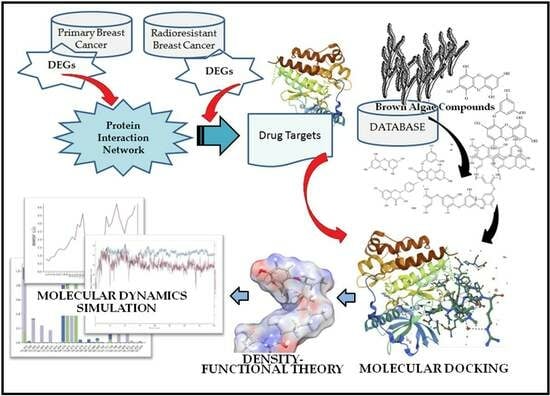
Network-Derived Radioresistant Breast Cancer Target with Candidate Inhibitors from Brown Algae: A Sequential Assessment from Target Selection to Quantum Chemical Calculation
Abstract
:1. Introduction
2. Results
2.1. Gene Expression Analysis of Radioresistant and Primary Breast Cancer Datasets
2.2. Protein Network Construction and Target Screening
2.3. Phytochemicals and Molecular Docking
2.4. Density Functional Theory of the Selected Compounds
2.4.1. Evaluation of Chemical Descriptors
| Quantum Chemical Descriptors | Nahocol-A1 | 4′-Chloro-2-hydroxyaurone | Mediterraneol B | (2E,6E,10E)-1-(2,5-Dihydroxy-3-methylphenyl)-13-hydroxy-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-5-one |
|---|---|---|---|---|
| Electron Volt (eV) | ||||
| Homo energy (EHOMO) | −5.7 | −4.81 | −4.01 | −4.21 |
| Lumo energy (ELUMO) | −0.86 | −1.6 | −1.35 | −1.45 |
| Ionization potential (I) | 5.7 | 4.81 | 4.01 | 4.21 |
| Electron affinity (A) | 0.86 | 1.6 | 1.35 | 1.45 |
| Energy gap (ΔE) | 4.84 | 3.2 | 2.66 | −2.76 |
| Chemical hardness (η) | 2.42 | 1.6 | 1.33 | 1.38 |
| Chemical softness (σ) | 0.41 | 0.62 | 0.75 | 0.72 |
| Electronegativity (χ) | 3.28 | 3.21 | 2.6 | 2.83 |
| Electrophilicity (ω) | 2.22 | 3.04 | 2.6 | 3.01 |
| Chemical potential (µ) | −3.28 | −3.21 | −1.75 | −2.83 |
2.4.2. Molecular Electrostatic Potential
2.5. MM-GBSA Calculation of the KIT-nahocol–A1 Complex
2.6. Molecular Dynamics Simulation of the Complex
3. Discussion
4. Materials and Methods
4.1. Data Collection and Differential Gene Expression Analysis
4.2. Protein Network Construction and Cluster Analysis
4.3. Screening of Therapeutic Targets and Phytochemicals
4.4. Comprehensive Marine Database Ligand Preparation
4.5. Protein Preparation and Molecular Docking
4.6. ADME Parameters
4.7. Density Functional Theory
4.8. Prime MM-GBSA Calculation
4.9. Molecular Dynamics Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Weigelt, B.; Glas, A.M.; Wessels, L.F.; Witteveen, A.T.; Peterse, J.L.; van’t Veer, L.J. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 15901–15905. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Rosso, K.; Nathanson, S.D. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J. Clin. Oncol. 2014, 5, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Turnbull, A.K.; Ward, C.; Meehan, J.; Martínez-Pérez, C.; Bonello, M.; Argyle, D. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat. Oncol. 2019, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, F.; Feng, Z.; Li, M.; Kong, L.; Huang, L.; Wei, Y.; Li, H.; Liu, F.; Zhang, H.; et al. Sunitinib facilitates metastatic breast cancer spreading by inducing endothelial cell senescence. Breast Cancer Res. BCR 2020, 22, 103. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Hu, S.; Pan, Y.; Zhang, J.; Tai, G.; Shao, C. GDF15 Contributes to Radioresistance by Mediating the EMT and Stemness of Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10911. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.; Li, Y.; Liu, K.; Zhao, Q.; Ouyang, L. Identification of claudin-6 as a molecular biomarker in pan-cancer through multiple omics integrative analysis. Front. Cell Dev. Biol. 2021, 9, 726656. [Google Scholar] [CrossRef]
- Srinivasan, E.; Nagaraj, S.; Rajendran, S.; Rajasekaran, R. Decoding novel therapeutic targets, biomarkers, and drug development strategies against neurodegenerative disorders—A multi-omics approach. Front. Comput. Neurosci. 2023, 17, 1227850. [Google Scholar] [CrossRef]
- Hao, X.; Cheng, S.; Jiang, B.; Xin, S. Applying multi-omics techniques to the discovery of biomarkers for acute aortic dissection. Front. Cardiovasc. Med. 2022, 9, 961991. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Isnansetyo, A.; Lutfia, F.N.L.; Nursid, M.; Susidarti, R.A. Cytotoxicity of fucoidan from three tropical brown algae against breast and colon cancer cell lines. Pharmacogn. J. 2017, 9, 14–20. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Deghady, A.M.; Hussein, R.K.; Alhamzani, A.G.; Mera, A. Density Functional Theory and Molecular Docking Investigations of the Chemical and Antibacterial Activities for 1-(4-Hydroxyphenyl)-3-phenylprop-2-en-1-one. Molecules 2021, 26, 3631. [Google Scholar] [CrossRef] [PubMed]
- Kafi, D.K.; Ayyash, A.N. Density functional theory and molecular docking study to lutein molecule for COVID-19 protease inhibitors. Appl. Nanosci. 2023, 13, 5477–5488. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Ahmad, S.; Zahiruddin, S.; Parveen, B.; Basist, P.; Parveen, A.; Parveen, R.; Ahmad, M. Indian Medicinal Plants and Formulations and Their Potential Against COVID-19-Preclinical and Clinical Research. Front. Pharmacol. 2021, 11, 578970. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Marín-Rubio, J.L.; Vela-Martín, L.; Fernández-Piqueras, J.; Villa-Morales, M. FADD in Cancer: Mechanisms of Altered Expression and Function, and Clinical Implications. Cancers 2019, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, J.; Li, F.; Ma, X.; Wu, F.; Miao, J.; Li, Q.; Wang, X.; Sun, R.; Yang, Y.; et al. POLR2A Promotes the Proliferation of Gastric Cancer Cells by Advancing the Overall Cell Cycle Progression. Front. Genet. 2021, 12, 688575. [Google Scholar] [CrossRef]
- Conte, A.; Valente, V.; Paladino, S.; Pierantoni, G.M. HIPK2 in cancer biology and therapy: Recent findings and future perspectives. Cell. Signal. 2022, 101, 110491. [Google Scholar] [CrossRef] [PubMed]
- Hagelkruys, A.; Sawicka, A.; Rennmayr, M.; Seiser, C. The biology of HDAC in cancer: The nuclear and epigenetic components. Histone Deacetylases Biol. Clin. Implic. 2011, 206, 13–37. [Google Scholar]
- Hu, H.; Yang, Y.; Ji, Q.; Zhao, W.; Jiang, B.; Liu, R.; Gong, Y. CRL4B catalyzes H2AK119 mon-oubiquitination and coordinates with PRC2 to promote tumorigenesis. Cancer Cell 2012, 22, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Peterson, Y.K.; Luttrell, L.M. The diverse roles of arrestin scaffolds in G protein–coupled receptor signaling. Pharmacol. Rev. 2017, 69, 256–297. [Google Scholar] [CrossRef]
- Güller, M.; Toualbi-Abed, K.; Legrand, A.; Michel, L.; Mauviel, A.; Bernuau, D.; Daniel, F. c-Fos overexpression increases the proliferation of human hepatocytes by stabilizing nuclear Cyclin D1. World J. Gastroenterol. 2008, 14, 6339–6346. [Google Scholar] [CrossRef]
- Sharma, S.; Gurudutta, G.U.; Satija, N.K.; Pati, S.; Afrin, F.; Gupta, P.; Tripathi, R.P. Stemcell c-KIT and HOXB4 genes: Critical roles and mechanisms in self-renewal, proliferation, and differentiation. Stem Cells Dev. 2006, 15, 755–778. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Schwartz, G.K. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Wragg, J.W.; Heath, V.L.; Bicknell, R. Sunitinib Treatment Enhances Metastasis of Innately Drug-Resistant Breast Tumors. Cancer Res. 2017, 77, 1008–1020. [Google Scholar] [CrossRef]
- Tang, X.; Tang, Q.; Yang, X.; Xiao, Z.A.; Zhu, G.; Yang, T.; Li, S. FN1 promotes prognosis and radioresistance in head and neck squamous cell carcinoma: From radioresistant HNSCC cell line to integrated bioinformatics methods. Front. Genet. 2022, 13, 1017762. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Lotia, S.; Pico, A.R.; Bader, G.D.; Ideker, T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Gade, A.C.; Murahari, M.; Pavadai, P.; Kumar, M.S. Virtual Screening of a Marine Natural Product Database for In Silico Identification of a Potential Acetylcholinesterase Inhibitor. Life 2023, 13, 1298. [Google Scholar] [CrossRef]
- Hage-Melim, L.I.D.S.; Federico, L.B.; de Oliveira, N.K.S.; Francisco, V.C.C.; Correia, L.C.; de Lima, H.B.; Gomes, S.Q.; Barcelos, M.P.; Francischini, I.A.G.; da Silva, C.H.T.P. Virtual screening, ADME/Tox predictions and the drug repurposing concept for future use of old drugs against the COVID-19. Life Sci. 2020, 256, 117963. [Google Scholar] [CrossRef]
- Dheeraj, G.; Kumar, P.; Apte, K.; Ashtekar, H.; Dixit, S.R. Molecular docking, ADME analysis, and pharmacophore modelling of benzoxazole fused azetidinone derivatives as antibreast cancer agents. Ann. Phytomed. 2023, 12, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Hari, G.; Pai, K.S.R. Molecular modeling piloted analysis for semicarbazone derivative of curcumin as a potent Abl-kinase inhibitor targeting colon cancer. 3 Biotech. 2021, 11, 506. [Google Scholar] [CrossRef]
- Sokkar, P.; Babu, A.; Kolandaswamy, A.; Daison, F.A.; Ramachandran, M. Effect of Substituents on the Photodynamic Action of Anthraquinones: EPR, Computational and In Vitro Studies. Photochem. Photobiol. 2022, 98, 1426–1433. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Perez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Daison, F.A.; Kumar, N.; Balakrishnan, S.; Venugopal, K.; Elango, S.; Sokkar, P. Molecular dynamics studies on the bacterial membrane pore formation by small molecule antimicrobial agents. J. Chem. Inf. Model. 2021, 62, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ansari, W.A.; Rab, S.O.; Saquib, M.; Sarfraz, A.; Hussain, M.K.; Akhtar, M.S.; Ahmad, I.; Khan, M.F. Pentafuhalol-B, a Phlorotannin from Brown Algae, Strongly Inhibits the PLK-1 Overexpression in Cancer Cells as Revealed by Computational Analysis. Molecules 2023, 28, 5853. [Google Scholar] [CrossRef] [PubMed]
- Sasitha, T.; John, W.J. Design, docking, and DFT investigations of 2,6-bis(3,4-dihydroxyphenyl)-3-phenethylpiperidin-4-one. Heliyon 2021, 7, e06127. [Google Scholar] [CrossRef] [PubMed]








| SI. No | Compounds | Glide Score (kcal/mol) |
|---|---|---|
| 1 | Nahocol-A1 | −8.56 |
| 2 | Ishigoside | −8.53 |
| 3 | 4′-chloro-2-hydroxyaurone | −8.51 |
| 4 | Mediterraneol B | −8.46 |
| 5 | (2E,6E,10E)-1-(2,5-dihydroxy-3-methylphenyl)-13-hydroxy-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-5-one | −8.31 |
| Complex | Δgbind (eV) | Δgcoul (eV) | ΔGH-Bond (eV) | Δglipo (eV) | ΔGGB (eV) | ΔGvdW (eV) |
|---|---|---|---|---|---|---|
| KIT–nahocol-A1 | −53.12 | −32.92 | −3.58 | −18.01 | −31.47 | −40.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, M.; Ahmad, S.F.; Emran, T.B.; Angulo-Bejarano, P.I.; Sharma, A.; Ahmed, S.S.S.J. Network-Derived Radioresistant Breast Cancer Target with Candidate Inhibitors from Brown Algae: A Sequential Assessment from Target Selection to Quantum Chemical Calculation. Mar. Drugs 2023, 21, 545. https://doi.org/10.3390/md21100545
Sivakumar M, Ahmad SF, Emran TB, Angulo-Bejarano PI, Sharma A, Ahmed SSSJ. Network-Derived Radioresistant Breast Cancer Target with Candidate Inhibitors from Brown Algae: A Sequential Assessment from Target Selection to Quantum Chemical Calculation. Marine Drugs. 2023; 21(10):545. https://doi.org/10.3390/md21100545
Chicago/Turabian StyleSivakumar, Mahema, Sheikh F. Ahmad, Talha Bin Emran, Paola Isabel Angulo-Bejarano, Ashutosh Sharma, and Shiek S. S. J. Ahmed. 2023. "Network-Derived Radioresistant Breast Cancer Target with Candidate Inhibitors from Brown Algae: A Sequential Assessment from Target Selection to Quantum Chemical Calculation" Marine Drugs 21, no. 10: 545. https://doi.org/10.3390/md21100545