Anti-Melanogenic Effects of Takifugu flavidus Muscle Hydrolysate in B16F10 Melanoma Cells and Zebrafish
Abstract
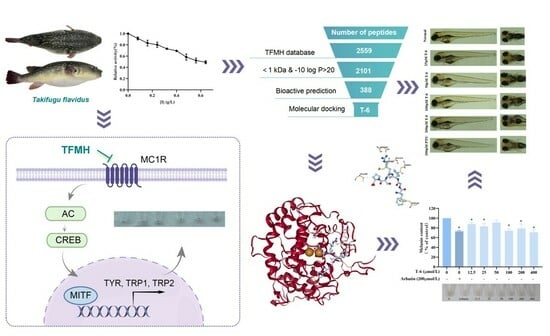
:1. Introduction
2. Results
2.1. Extraction of TFMH
2.1.1. Selection of the Optimal Protease
2.1.2. Single-Factor Experiments
2.1.3. Model Fitting and Statistical Evaluation
2.1.4. Validation of the Model
2.1.5. Molecular Weight Distribution of TFMH
2.2. Inhibitory Effect of TFMH on TYR Enzyme Activity
2.3. Inhibitory Activity of TFMH on Melanogenesis in B16F10 Melanoma Cells and Mechanism of Action
2.4. Effect of TFMH on Melanogenesis in Zebrafish
2.5. Safety Assessment of TFMH
2.6. Identification and Evaluation of Bioactive Peptides for Melanogenesis Inhibition in TFMH
2.7. Analysis of the Interaction between T-6 and TYR
2.8. Validation of the Inhibitory Efficacy against Melanin Production
3. Discussion
4. Materials and Methods
4.1. Preparation of TFMH
4.2. Optimization of the TFMH Extraction Process
4.2.1. Determination of the mTYR Activity Inhibition Rate by Enzymatic Hydrolysate
4.2.2. Protease Screening
4.2.3. Single-Factor Experiments for Enzymatic Extraction of TFMH
4.2.4. Optimization of the Enzymatic Extraction Process for TFMH by Response Surface Methodology
4.2.5. Molecular Weight Distribution of TFMH
4.3. Inhibition Effect of TFMH on mTYR Diphenolase Activity
4.4. Cell Culturing
4.5. Cell Viability Assay
4.6. Melanin Content and TYR Activity Measurement in B16F10 Cells
4.7. Western Blot Analysis for Detection of Cell Melanin Signal Pathway Protein Expression
4.8. Determination of Zebrafish Embryo Survival Rate
4.9. Zebrafish Embryo Exposure
4.10. Determination of Melanin Content and TYR Activity in Zebrafish Embryos
4.11. Mouse Acute Toxicity Test
4.12. Nano-HPLC-MS/MS Identification of Peptide Sequences
4.13. Virtual Screening of Peptides
4.14. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Krasowska, D.; Malek, A.; Kurzepa, J.; Kapka-Skrzypczak, L.; Krasowska, D.; Kurzepa, J. Melanin-The Eminence Grise of Melanoma and Parkinson’s Disease Development. Cancers 2023, 15, 5541. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.W. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J. Dermatol. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of Kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29, 244s–273s. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Joompang, A.; Jangpromma, N.; Choowongkomon, K.; Payoungkiattikun, W.; Tankrathok, A.; Viyoch, J.; Luangpraditkun, K.; Klaynongsruang, S. Evaluation of tyrosinase inhibitory activity and mechanism of Leucrocin I and its modified peptides. J. Biosci. Bioeng. 2020, 130, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Su, X.R.; Liu, S.S.; Yang, S.S.; Jiang, C.Y.; Zhang, Y.; Zhang, S. Zebrafish phosvitin-derived peptide Pt5 inhibits melanogenesis via cAMP pathway. Fish. Physiol. Biochem. 2017, 43, 517–525. [Google Scholar] [CrossRef]
- Meng, J.; Liu, J.; Lu, J.; Jiang, P.; Bai, Y.; Liu, X.; Li, S. Isolation, identification, and preparation of tyrosinase inhibitory peptides from Pinctada martensii meat. Biotechnol. Lett. 2023, 45, 1495–1511. [Google Scholar] [CrossRef]
- He, Y.; Suyama, T.L.; Kim, H.; Glukhov, E.; Gerwick, W.H. Discovery of Novel Tyrosinase Inhibitors from Marine Cyanobacteria. Front. Microbiol. 2022, 13, 912621. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Q.; Zhang, H.; Wang, L.; Zhang, F.; Yang, C.; Song, L. Draft sequencing and analysis of the genome of pufferfish Takifugu flavidus. DNA Res. 2014, 21, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Zhang, G.Y.; Zhu, Y.Z.; Liu, J.Z.; Zang, W.L. Effects of temperature on fertilized eggs and larvae of tawny puffer Takifugu flavidus. Aquac. Res. 2010, 41, 1741–1747. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Yi, R.; Bai, K.; Wang, G.; Tan, R.; Sun, S.; Xu, N. Electrodialysis Extraction of Pufferfish Skin (Takifugu flavidus): A Promising Source of Collagen. Mar. Drugs 2019, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Lajis, A.F.B. A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine. Medicina 2018, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Vudhya Gowrisankar, Y.; Wang, L.W.; Zhang, Y.Z.; Chen, X.Z.; Huang, P.J.; Yen, H.R.; Yang, H.L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox. Biol. 2021, 44, 102007. [Google Scholar] [CrossRef] [PubMed]
- GB/T 15193.3-2014; Standardization Administration of China. National Food Safety Standard-Acute Toxicity Test. Standards Press of China: Beijing, China, 2014.
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Talebi, M.; Majidi, K.; Bassam, K.; Abdi, M.; Daneshvar, M.; Moayedi, S.S.; Pourhesabi, S.; Attarroshan, M.; Boumi, S.; Kabiri, M.; et al. Synthesis, biological evaluation, and molecular docking analysis of novel 1, 3, 4-thiadiazole -based kojic acid derivatives as tyrosinase inhibitors. J. Mol. Struct. 2022, 1268, 133707. [Google Scholar] [CrossRef]
- Asadzadeh, A.; Sirous, H.; Pourfarzam, M.; Yaghmaei, P.; Afshin, F. In vitro and in silico studies of the inhibitory effects of some novel kojic acid derivatives on tyrosinase enzyme. Iran. J. Basic. Med. Sci. 2016, 19, 132–144. [Google Scholar]
- Robin, X.; Studer, G.; Durairaj, J.; Eberhardt, J.; Schwede, T.; Walters, W.P. Assessment of protein-ligand complexes in CASP15. Proteins 2023, 91, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Jalkute, C.B.; Barage, S.H.; Dhanavade, M.J.; Sonawane, K.D. Molecular dynamics simulation and molecular docking studies of Angiotensin converting enzyme with inhibitor lisinopril and amyloid Beta Peptide. Protein J. 2013, 32, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Darden, T.A.; Cheatham, T.E.I.; Simmerling, C.L.; Kollman, P.A. AMBER 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Feng, Y.X.; Wang, Z.C.; Chen, J.X.; Li, H.R.; Wang, Y.B.; Ren, D.F.; Lu, J. Separation, identification, and molecular docking of tyrosinase inhibitory peptides from the hydrolysates of defatted walnut (Juglans regia L.) meal. Food Chem. 2021, 353, 129471. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Bang, J.S.; Choi, Y.J.; Choung, S.Y. Anti-melanogenic effects of oyster hydrolysate in UVB-irradiated C57BL/6J mice and B16F10 melanoma cells via downregulation of cAMP signaling pathway. J. Ethnopharmacol. 2019, 229, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Ben Mlouka, M.A.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Moon, K.M.; Yang, J.H.; Lee, M.K.; Kwon, E.B.; Baek, J.; Hwang, T.; Kim, J.I.; Lee, B. Maclurin Exhibits Antioxidant and Anti-Tyrosinase Activities, Suppressing Melanogenesis. Antioxidants 2022, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Lee, K.J.; Kim, Y.; Kim, M.; Ju, H.M.; Kim, M.J.; Choi, D.H.; Choi, J.; Kim, S.; Kang, D.; et al. A Basic Domain-Derived Tripeptide Inhibits MITF Activity by Reducing its Binding to the Promoter of Target Genes. J. Investig. Dermatol. 2021, 141, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Qomaladewi, N.P.; Kim, M.Y.; Cho, J.Y. Rottlerin Reduces cAMP/CREB-Mediated Melanogenesis via Regulation of Autophagy. Int. J. Mol. Sci. 2019, 20, 2081. [Google Scholar] [CrossRef]
- Li, H.L.; Li, M.J.; Xiong, G.Q.; Cai, J.; Liao, T.; Zu, X.Y. Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway. Nutrients 2023, 15, 2449. [Google Scholar] [CrossRef]
- Seo, G.Y.; Ha, Y.; Park, A.H.; Kwon, O.W.; Kim, Y.J. Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, J.; Palade, V. MSLDOCK: Multi-Swarm Optimization for Flexible Ligand Docking and Virtual Screening. J. Chem. Inf. Model. 2021, 61, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Azami, F.; Tazikeh, L.E.; Mahmood-Janlou, J.M.A. Kojic Acid Effect on the Inhibitory Potency of Tyrosinase. J. Chem. Health Risks 2016, 7, 147–155. [Google Scholar]
- Hassani, S.; Haghbeen, K.; Fazli, M. Non-specific binding sites help to explain mixed inhibition in mushroom tyrosinase activities. Eur. J. Med. Chem. 2016, 122, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Roselan, M.A.; Zakaria, N.; Faujan, N.H.; Mohammad Latif, M.A.; Mohd Faudzi, S.M.; Ab Hadi, H.; Ashari, S.E. In vitro cytotoxicity assay, mushroom tyrosinase inhibitory activity and release analysis of kojic monooleate nanodelivery system and in silico molecular docking study against 2Y9X target enzyme. J. Drug Deliv. Sci. Technol. 2021, 66, 102764. [Google Scholar] [CrossRef]
- Javed, A.; Alam, M.B.; Naznin, M.; Shafique, I.; Kim, S.; Lee, S.H. Tyrosinase inhibitory activity of Sargassum fusiforme and characterisation of bioactive compounds. Phytochem. Anal. 2023. [Google Scholar] [CrossRef]
- Canedo, A.; Rocha, T.L. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: Historical review, current status and trends. Sci. Total Environ. 2021, 762, 144084. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-W.; Kuo, I.C.; Syu, L.-L.; Lee, T.-H.; Cheng, C.-H.; Mei, H.-C.; Lee, C.-K. Assessing the skin-whitening property of plant extracts from taiwanese species using zebrafish as a rapid screening platform. Arab. J. Chem. 2023, 16, 105035. [Google Scholar] [CrossRef]
- Agalou, A.; Thrapsianiotis, M.; Angelis, A.; Papakyriakou, A.; Skaltsounis, A.L.; Aligiannis, N.; Beis, D. Identification of novel melanin synthesis inhibitors from Crataegus pycnoloba using an in vivo zebrafish phenotypic assay. Front. Pharmacol. 2018, 9, 265. [Google Scholar] [CrossRef]
- Ouyang, T.; Yin, H.; Yang, J.; Liu, Y.; Ma, S. Tissue regeneration effect of betulin via inhibition of ROS/MAPKs/NF-ĸB axis using zebrafish model. Biomed. Pharmacother. 2022, 153, 113420. [Google Scholar] [CrossRef]
- Kasica, N.; Jakubowski, P.; Kaleczyc, J. P-Glycoprotein Inhibitor Tariquidar Plays an Important Regulatory Role in Pigmentation in Larval Zebrafish. Cells 2021, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Jingxin, T.; Yanlin, L.; Ronghui, Z. Optimization of Ultrasonic Assisted Extraction of Flavonoid from Pomelo Peel by Response Surface Methodology; Shandong Chemical Industry: Shouguang, China, 2018; Volume 47, pp. 8–12. [Google Scholar]
- GB/T 22729-2008; Standardization Administration of China. Oligopeptides Powder of Marine Fish. Standards Press of China: Beijing, China, 2008.
- Chai, W.M.; Chen, C.M.; Gao, Y.S.; Feng, H.L.; Ding, Y.M.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Structural analysis of proanthocyanidins isolated from fruit stone of Chinese hawthorn with potent antityrosinase and antioxidant activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Lee, H.J.; Park, D.; Jeong, H.O.; Park, J.Y.; Park, Y.J.; Lee, K.J.; Lee, J.Y.; Moon, H.R.; Chung, H.Y. Molecular docking studies of (1E,3E,5E)-1,6-Bis(substituted phenyl)hexa-1,3,5-triene and 1,4-Bis(substituted trans-styryl)benzene analogs as novel tyrosinase inhibitors. Biol. Pharm. Bull. 2013, 36, 55–65. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, R.-J.; Chen, Y.-M.; Ye, X.; Chen, Q.-X.; Shen, D.-Y.; Wang, Q. Synthesis of caffeic acid ester morpholines and their activation effects on tyrosinase. Process Biochem. 2017, 62, 91–98. [Google Scholar] [CrossRef]












| Run | A | B | C | Inhibition Rate |
|---|---|---|---|---|
| Temperature | Enzyme Amount | pH | (IR/%) | |
| 1 | 0 | 1 | −1 | 80.31 |
| 2 | −1 | −1 | 0 | 75.69 |
| 3 | −1 | 0 | 1 | 78.58 |
| 4 | 0 | 0 | 0 | 92.96 |
| 5 | 0 | 0 | 0 | 92.88 |
| 6 | 1 | 0 | −1 | 75.06 |
| 7 | 0 | 0 | 0 | 88.21 |
| 8 | −1 | 1 | 0 | 77.76 |
| 9 | 0 | 0 | 0 | 90.66 |
| 10 | −1 | 0 | −1 | 77.81 |
| 11 | 0 | −1 | −1 | 78.84 |
| 12 | 0 | −1 | 1 | 77.54 |
| 13 | 1 | −1 | 0 | 75.78 |
| 14 | 0 | 0 | 0 | 90.78 |
| 15 | 0 | 1 | 1 | 74.34 |
| 16 | 1 | 1 | 0 | 75.88 |
| 17 | 1 | 0 | 1 | 76.05 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Variance | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 725.41 | 9 | 80.60 | 19.53 | 0.0004 | ** |
| A | 6.25 | 1 | 6.25 | 1.51 | 0.2583 | |
| B | 0.024 | 1 | 0.024 | 5.863 × 10−3 | 0.9411 | |
| C | 3.80 | 1 | 3.80 | 0.92 | 0.3696 | |
| AB | 0.97 | 1 | 0.97 | 0.24 | 0.6426 | |
| AC | 0.012 | 1 | 0.012 | 2.932 × 10−3 | 0.9583 | |
| BC | 5.45 | 1 | 5.45 | 1.32 | 0.2882 | |
| A2 | 259.56 | 1 | 259.56 | 62.89 | <0.0001 | ** |
| B2 | 204.49 | 1 | 204.49 | 49.54 | 0.0002 | ** |
| C2 | 170.93 | 1 | 170.93 | 41.41 | 0.0004 | ** |
| Residual | 28.89 | 7 | 4.13 | |||
| Lack of Fit | 13.62 | 3 | 4.54 | 1.19 | 0.4199 | |
| Pure Error | 15.28 | 4 | 3.82 | |||
| Total | 754.30 | 16 | ||||
| R2 = 96.17%, R2Adj = 91.24 | ||||||
| Sequence Number | Peptide Sequence | Grid Score (kcal/mol) |
|---|---|---|
| T-1 | SGFPRHR | −153.2497 |
| T-2 | LSGFPRHR | −153.2468 |
| T-3 | IRWR | −132.6575 |
| T-4 | ARWNPAPGP | −126.1295 |
| T-5 | WGPDPR | −125.9351 |
| T-6 | FGFRSP | −118.9725 |
| T-7 | DWPDGRG | −113.1157 |
| T-8 | MGRWL | −111.5497 |
| T-9 | FFRI | −110.2282 |
| T-10 | FMRF | −109.5137 |
| T-11 | LWDR | −104.4215 |
| T-12 | YPRF | −103.6229 |
| T-13 | FIRF | −102.5375 |
| T-14 | FNRTPIGW | −126.8967 |
| T-15 | IRFR | −128.7539 |
| Number | Protease Species | Optimal pH | Optimal Temperature (°C) |
|---|---|---|---|
| 1 | Trypsin | 7.5 | 37 |
| 2 | Acid Protease | 4 | 50 |
| 3 | Neutral Protease | 6 | 50 |
| 4 | Papain | 6.5 | 60 |
| 5 | Alkaline Protease | 8.5 | 55 |
| Level | Factor | ||
|---|---|---|---|
| Temperature/°C (A) | Enzyme Amount/(U/g) (B) | pH (C) | |
| −1 | 32 | 2000 | 6.5 |
| 0 | 37 | 3000 | 7 |
| 1 | 42 | 4000 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Chen, B.; Qu, S.; Liu, S.; Yang, X.; Qiao, K.; Su, Y.; Liu, Z.; Chen, X.; Liu, Z.; et al. Anti-Melanogenic Effects of Takifugu flavidus Muscle Hydrolysate in B16F10 Melanoma Cells and Zebrafish. Mar. Drugs 2024, 22, 206. https://doi.org/10.3390/md22050206
Hu J, Chen B, Qu S, Liu S, Yang X, Qiao K, Su Y, Liu Z, Chen X, Liu Z, et al. Anti-Melanogenic Effects of Takifugu flavidus Muscle Hydrolysate in B16F10 Melanoma Cells and Zebrafish. Marine Drugs. 2024; 22(5):206. https://doi.org/10.3390/md22050206
Chicago/Turabian StyleHu, Jinjin, Bei Chen, Shuaijie Qu, Shuji Liu, Xiaoyu Yang, Kun Qiao, Yongchang Su, Zhihui Liu, Xiaoe Chen, Zhiyu Liu, and et al. 2024. "Anti-Melanogenic Effects of Takifugu flavidus Muscle Hydrolysate in B16F10 Melanoma Cells and Zebrafish" Marine Drugs 22, no. 5: 206. https://doi.org/10.3390/md22050206