Hydration Performance of Magnesium Potassium Phosphate Cement Using Sodium Alginate as a Candidate Retarder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
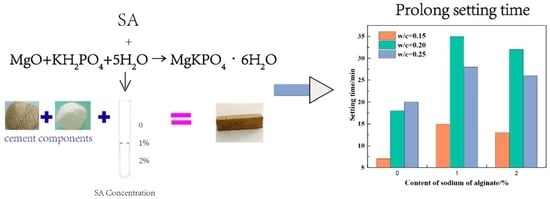
3.1. Setting Time
3.2. Compressive Strength
3.3. Hydration Products
3.4. Microstructures
4. Conclusions
- (1)
- Sodium alginate presented dramatical retarding effects on MKPCs in the range of 0% to 2% (by mass of water). One percent of sodium alginate by mass of water could extend the setting time of MKPCs from 15 min to 35 min, thus presenting a better retarding effect than borax and producing higher early strength of MKPCs.
- (2)
- The presence of sodium alginate led to more residual MgO at the very early stage, but less well-crystallized KH2PO4 compared to the MKPCs containing no sodium alginate. The presence of sodium alginate seemed to assist the dissolution of KH2PO4.
- (3)
- The compressive strength of the MKPCs increased when the concentration of sodium alginate was within 1%, regardless of the w/c ratio. The effect of sodium alginate on the compressive strength of MKPCs seemed to differ with that seen upon introducing borax, which was most likely caused by the adsorption and storage capacity of water by the sodium alginate, in addition to the presence of sodium alginate altering the pH environment of MKPCs.
- (4)
- The addition of sodium alginate at a content of no more than 1% could contribute to the reaction of the MKPC system, but a reverse effect would be found with more than 1% sodium alginate.
- (5)
- No new phases such as Na-containing assemblages were clearly observed by SEM analysis; however, the assumption that sodium alginate probably took part in the reaction of MKPCs cannot excluded, and further evidence should be obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| MPC | magnesium phosphate cement |
| MKPC | magnesium potassium phosphate cement |
| SA | sodium alginate |
| MgO | dead burnt magnesia. |
| KDP | potassium dihydrogen phosphate. |
| w/c | water-to-cement (including MgO and KDP) ratio by mass |
References
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Enhancement of magnesium phosphate cement solidification of Pb2+ by K-struvite whisker in lead-contaminated solution. J. Clean. Prod. 2021, 320, 128848. [Google Scholar] [CrossRef]
- You, C.; Qian, J.; Qin, J.; Wang, H.; Wang, Q.; Ye, Z. Effect of early hydration temperature on hydration product and strength development of magnesium phosphate cement (MPC). Cem. Concr. Res. 2015, 78, 179–189. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B.; Maierdan, Y. Influence of supplementary materials on the early age hydration reactions and microstructural progress of magnesium phosphate cement matrices. J. Clean. Prod. 2022, 333, 130086. [Google Scholar] [CrossRef]
- Coumes, C.C.D.; Rousselet, A.; Xu, B.; Mercier, C.A.; Gauffinet, S. Investigation of aluminum nitrate as a set retarder of magnesium potassium phosphate cement: Mechanisms involved in diluted suspension. Cem. Concr. Res. 2021, 150, 106608. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Zhou, H.; He, F.; Li, Y.; Ren, W.; Du, Y. Influencing mechanism of sodium tripolyphosphate on the rheological properties of magnesium phosphate cement. Powder Technol. 2021, 387, 406–414. [Google Scholar] [CrossRef]
- Langton, G.; Stefanko, D. Magnesium Mono Potassium Phosphate Grout For P-Reactor Vessel In-Situ Decomissioning. Off. Sci. Tech. Inf. Tech. Rep. 2011, 42. [Google Scholar] [CrossRef] [Green Version]
- Yun-Tao, L.I.; Yan, H.; Wang, H.T.; Wang, Q.; Zhao, S.X. Effect of Paraffin/Expanded Graphite Composite Phase Change Materials on the Hydration Performances of Magnesium Phosphate Cement. Bull. Chin. Ceram. Soc. 2016, 9, 3007–3013. [Google Scholar] [CrossRef]
- Yuntao, L.I.; Yan, H.; Wang, Q.; Wang, H.; Zhao, S. Effect of stearic acid composite phase change material on thermal properties of magnesium phosphate cement. New Build. Mater. 2017, 44, 122–127. [Google Scholar]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterisation of magnesium potassium phosphate cements blended with fly ash and ground granulated blast furnace slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Lv, L.; Deng, M.; Qian, J. Influence of fly ash and metakaolin on the microstructure and compressive strength of magnesium potassium phosphate cement paste. Cem. Concr. Res. 2018, 111, 116–129. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of wollastonite on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of magnesium phosphate cement hydration in diluted suspension and its retardation by boric acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Hejazi, S.A.S.; Zhao, C.; Xie, M. The effect of low temperature phase change material of hydrated salt on the performance of magnesium phosphate cement. Constr. Build. Mater. 2017, 149, 272–278. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Li, Y.; Shi, T. Effects of fly ash, retarder and calcination of magnesia on properties of magnesia–phosphate cement. Adv. Cem. Res. 2015, 27, 373–380. [Google Scholar]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C.K.; Qiao, F. Setting and strength development of magnesium phosphate cement paste. Adv. Cem. Res. 2009, 21, 175–180. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Ren, H.; Ding, Z.; Ji, M.; Yan, Y. Developing a novel magnesium calcium phosphate/sodium alginate composite cement with high strength and proper self-setting time for bone repair. J. Biomater. Appl. 2021, 36, 8853282211021535. [Google Scholar] [CrossRef]
- Wei, L.; Hong, T.; Liu, H.; Chen, T. The effect of sodium alginate on struvite crystallization in aqueous solution: A kinetics study. J. Cryst. Growth 2017, 473, 60–65. [Google Scholar] [CrossRef]
- Test Method for Water Consumption, Setting Time and Stability of Cement Standard Consistency; GB/T 1346; China Standards Press: Beijing, China, 2011.
- Magnesium Phosphate Repair Mortar; JC/T 2537–2019; China Building Materials Industry Press: Beijing, China, 2019.
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Li, Z. Influence of magnesia-to-phosphate molar ratio on microstructures, mechanical properties and thermal conductivity of magnesium potassium phosphate cement paste with large water-to-solid ratio. Cem. Concr. Res. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Fan, S.; Chen, B. Experimental study of phosphate salts influencing properties of magnesium phosphate cement. Constr. Build. Mater. 2014, 65, 480–486. [Google Scholar] [CrossRef]
- Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2013; p. C191-13.
- Li, Y.; Chen, B. Factors that affect the properties of magnesium phosphate cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of boric acid content on the properties of magnesium phosphate cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Feng, H.; Li, Z.; Wang, W.; Liu, G.; Gao, D. Deflection hardening behaviour of ductile fibre reinforced magnesium phosphate cement-based composite. Cem. Concr. Compos. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Jia, X.-W.; Luo, J.-Y.; Zhang, W.-X.; Tang, M.-H.; Qian, J.-S.; Wang, P.; Li, J.-M. Reaction characteristics and compressive strength of magnesia-phosphate cement at negative temperatures. Constr. Build. Mater. 2021, 305, 124819. [Google Scholar] [CrossRef]
- Xue, M.; Cao, J.; Jiang, J. Influence of Borax on Properties of Magnesium Phosphate Cement and Its Microscopic Mechanism. J. Logist. Eng. Univ. 2011, 27, 52–55. [Google Scholar]
- Hongyan, M. Mercury intrusion porosimetry in concrete technology: Tips in measurement, pore structure parameter acquisition and application. J. Porous Mater. 2014, 21, 207–215. [Google Scholar]










| No. | MgO/g | KDP/g | SA Concentration/% | w/c Ratio |
|---|---|---|---|---|
| 1 | 108 | 92 | 0% | 0.15 |
| 2 | 108 | 92 | 0% | 0.20 |
| 3 | 108 | 92 | 0% | 0.25 |
| 4 | 108 | 92 | 1% | 0.15 |
| 5 | 108 | 92 | 1% | 0.20 |
| 6 | 108 | 92 | 1% | 0.25 |
| 7 | 108 | 92 | 2% | 0.15 |
| 8 | 108 | 92 | 2% | 0.20 |
| 9 | 108 | 92 | 2% | 0.25 |
| A | 108 | 92 | 0% | 10 |
| B | 108 | 92 | 1% | 10 |
| C | 108 | 92 | 2% | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Fang, B.; Zhang, G.; Guo, J.; Liu, R. Hydration Performance of Magnesium Potassium Phosphate Cement Using Sodium Alginate as a Candidate Retarder. Materials 2022, 15, 943. https://doi.org/10.3390/ma15030943
Yang Y, Fang B, Zhang G, Guo J, Liu R. Hydration Performance of Magnesium Potassium Phosphate Cement Using Sodium Alginate as a Candidate Retarder. Materials. 2022; 15(3):943. https://doi.org/10.3390/ma15030943
Chicago/Turabian StyleYang, Yuanquan, Bodong Fang, Guanhua Zhang, Jinbo Guo, and Runqing Liu. 2022. "Hydration Performance of Magnesium Potassium Phosphate Cement Using Sodium Alginate as a Candidate Retarder" Materials 15, no. 3: 943. https://doi.org/10.3390/ma15030943