Synthesis and Characterization of Green ZnO@polynaniline/Bentonite Tripartite Structure (G.Zn@PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
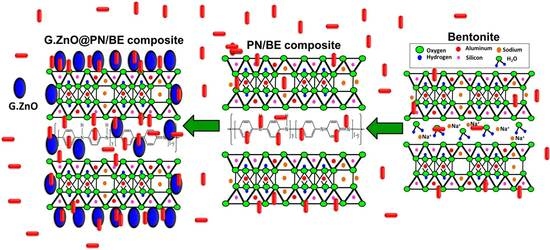
2.2. Synthesis of Green ZnO@polyaniline/Bentonite Composite (G.Zn@PN/BE)
2.3. Analytical Techniques
2.4. Adsorption Studies
3. Results and Discussion
3.1. Characterization of G.Zn@PN/BE Structure
3.2. Retention Results
3.2.1. Retention pH
3.2.2. Retention Time Interval
3.2.3. Kinetic Studies
Intra-Particle Diffusion Behavior
Kinetic Modeling
3.2.4. As (V) Concentration
3.2.5. Classic Isotherm Models
3.2.6. Advanced Isotherm Models
Steric Properties
- Number of adsorbed As (V) ions per site (n)
- Density of the active sites (Nm)
- The saturation As (V) adsorption capacity (Qsat)
Energetic Properties
- Adsorption energy
- Thermodynamic functions
- Internal energy and free enthalpy
- Entropy
3.2.7. Effect of Coexisting Cations and Anions
3.2.8. Recyclability
3.2.9. Comparison Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Abukhadra, M.R. Insight into the role of the zeolitization process in enhancing the adsorption performance of kaolinite/diatomite geopolymer for effective retention of Sr (II) ions; batch and column studies. J. Environ. Manag. 2021, 294, 112984. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, B.; Liu, Y.; Zhou, F.; Li, D.; Zhang, Z. Gamma-FeOOH based hierarchically porous zeolite monoliths for As (V) removal: Characterisation, adsorption and response surface methodology. Microporous Mesoporous Mater. 2020, 308, 110518. [Google Scholar] [CrossRef]
- Javaheri, F.; Kheshti, Z.; Ghasemi, S.; Altaee, A. Enhancement of Cd2+ removal from aqueous solution by multifunctional mesoporous silica: Equilibrium isotherms and kinetics study. Sep. Purif. Technol. 2019, 224, 199–208. [Google Scholar] [CrossRef]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Y.; Fu, X.; Lu, H.; Zhang, J. A novel layer-layer crossed structure of bentonite/g-C3N4 for enhanced photocatalytic oxidation of arsenic (III) in a wide pH range. Surf. Interfaces 2021, 26, 101365. [Google Scholar] [CrossRef]
- Mutar, R.F.; Saleh, M.A. Optimization of arsenic ions adsorption and removal from hospitals wastewater by nano-bentonite using central composite design. Mater. Today Proc. 2021, 60, 1248–1256. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.W.; Bhatnagar, A.; Song, H. Adsorption of As (V) and Ni (II) by Fe-Biochar composite fabricated by co-pyrolysis of orange peel and red mud. Environ. Res. 2020, 188, 109809. [Google Scholar] [CrossRef]
- Jumah, M.N.B.; Eid, M.H.; AL-Huqail, A.A.; Mohammad, M.A.; Bin-Murdhi, N.S.; Abu-Taweel, G.M.; Altoom, N.; Allam, A.A.; AbuKhadra, M.R. Enhanced remediation of As (V) and Hg (II) ions from aqueous environments using β-cyclodextrin/MCM-48 composite: Batch and column studies. J. Water Process Eng. 2021, 42, 102118. [Google Scholar]
- Nandi, D.; Ghosh, S.K.; Ghosh, A.; Siengchin, S.; Roy, A.; Gupta, K.; Parameswaranpillai, J.; Bhowmick, A.K.; Ghosh, U.C. Arsenic removal from water by graphene nanoplatelets prepared from nail waste: A physicochemical study of adsorption based on process optimization, kinetics, isotherm and thermodynamics. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100564. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic (V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef]
- Penke, Y.K.; Yadav, A.K.; Malik, I.; Tyagi, A.; Ramkumar, J.; Kar, K.K. Insights of arsenic (III/V) adsorption and electrosorption mechanism onto multi synergistic (redox-photoelectrochemical-ROS) aluminum substituted copper ferrite impregnated rGO. Chemosphere 2021, 267, 129246. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, D.; Ponte, R.; Zhang, N.; Nakamura, T.; Kawamoto, T. Synthesis and characterization of mixed Co-Zn-ZIF for arsenic (V) adsorption. Inorg. Chim. Acta 2020, 502, 119311. [Google Scholar] [CrossRef]
- Mahato, B.N.; Krithiga, T.; Thangam, M.M. Rapid adsorption of As (V) from aqueous solution by ZnO embedded in mesoporous aluminosilicate nanocomposite adsorbent: Parameter optimization, kinetic, and isotherms studies. Surf. Interfaces 2021, 23, 100636. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Roos, W.D. Enhanced adsorption of As (V) and Mn (VII) from industrial wastewater using multi-walled carbon nanotubes and carboxylated multi-walled carbon nanotubes. Chemosphere 2020, 254, 126780. [Google Scholar] [CrossRef]
- Tan, G.; Mao, Y.; Wang, H.; Xu, N. A comparative study of arsenic (V), tetracycline and nitrate ions adsorption onto magnetic biochars and activated carbon. Chem. Eng. Res. Des. 2020, 159, 582–591. [Google Scholar] [CrossRef]
- Ramirez-Muñiz, K.; Perez-Rodriguez, F.; Rangel-Mendez, R. Adsorption of arsenic onto an environmental friendly goethite-polyacrylamide composite. J. Mol. Liq. 2018, 264, 253–260. [Google Scholar] [CrossRef]
- Wang, L.; Muhammad, H.; Laipan, M.; Fan, X.; Guo, J.; Li, Y. Enhanced removal of Cr (VI) and Mo (VI) from polluted water using L-cysteine doped polypyrrole/bentonite composite. Appl. Clay Sci. 2022, 217, 106387. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Yang, R.; Zhou, G.; Yu, P.; Sun, L.; Hao, T.; Wang, J.; Liu, Y. Novel environment-friendly magnetic bentonite nanomaterials functionalized by carboxymethyl chitosan and 1-(2-pyridinylazo)-2-naphthaleno for adsorption of Sc (III). Appl. Surf. Sci. 2021, 566, 150644. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Eid, M.H.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T. Insight into chitosan/mesoporous silica nanocomposites as eco-friendly adsorbent for enhanced retention of U (VI) and Sr (II) from aqueous solutions and real water. Int. J. Biol. Macromol. 2021, 173, 435–444. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Tu, Y.; Muhammad, H.; Fan, X.; Cao, G.; Laipan, M. Polypyrrole modified bentonite nanocomposite and its application in high-efficiency removal of Cr (VI). J. Environ. Chem. Eng. 2021, 9, 106631. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption Behaviors and Mechanisms of Fe/Mg Layered Double Hydroxide Loaded on Bentonite on Cd (II) and Pb (II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Landge, V.K.; Sonawane, S.H.; Sivakumar, M.; Sonawane, S.S.; Babu, G.U.B.; Boczkaj, G. S-scheme heterojunction Bi2O3-ZnO/Bentonite clay composite with enhanced photocatalytic performance. Sustain. Energy Technol. Assess. 2021, 45, 101194. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Luqman, M. Insight into carbohydrate polymers (chitosan and 2-hydroxyethyl methacrylate/methyl methacrylate) intercalated bentonite-based nanocomposites as multifunctional and environmental adsorbents for methyl parathion pesticide. Int. J. Biol. Macromol. 2021, 167, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Abd Elkader, Y.; Abd El-Halim, M.R. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rend. Lincei Sci. Fis. Nat. 2018, 29, 141–154. [Google Scholar] [CrossRef]
- Sayed, M.A.; Abukhadra, M.R.; Salam, M.A.; Yakout, S.M.; Abdeltawab, A.A.; Aziz, I.M. Photocatalytic hydrogen generation from raw water using zeolite/polyaniline@Ni2O3 nanocomposite as a novel photo-electrode. Energy 2019, 187, 115943. [Google Scholar] [CrossRef]
- Bober, P.; Stejskal, J.; Spírková, M.; Trchová, M.; Varga, M.; Proke, J. Conducting polyaniline–montmorillonite composites. Synth. Met. 2010, 160, 2596–2604. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Shaban, M.; Sayed, F.; Saad, I. Efficient photocatalytic removal of safarnin-O dye pollutants from water under sunlight using synthetic bentonite/polyaniline@Ni2O3 photocatalyst of enhanced properties. Environ. Sci. Pollut. Res. 2018, 25, 33264–33276. [Google Scholar] [CrossRef]
- Khushboo; Kaur, M.; Jeet, K. Mechanistic insight into adsorption and photocatalytic potential of magnesium ferrite-bentonite nanocomposite. J. Photochem. Photobiol. A 2022, 425, 113717. [Google Scholar]
- Uddin, M.K.; Mashkoor, F.; AlArifi, I.M.; Nasar, A. Simple one-step synthesis process of novel MoS2@ bentonite magnetic nanocomposite for efficient adsorption of crystal violet from aqueous solution. Mater. Res. Bull. 2021, 139, 111279. [Google Scholar] [CrossRef]
- Kaur, M.; Ubhi, M.K.; Grewal, J.K.; Singh, D. Insight into the structural, optical, adsorptive, and photocatalytic properties of MgFe2O4-bentonite nanocomposites. J. Phys. Chem. Solids 2022, 154, 110060. [Google Scholar] [CrossRef]
- Długosz, O.; Wąsowicz, N.; Szostak, K.; Banach, M. Photocatalytic properties of coating materials enriched with bentonite/ZnO/CuO nanocomposite. Mater. Chem. Phys. 2021, 260, 124150. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. Environ. Manag. Today 2020, 258, 110043. [Google Scholar] [CrossRef] [PubMed]
- Nazarkovsky, M.; Czech, B.; Żmudka, A.; Bogatyrov, V.M.; Artiushenko, O.; Zaitsev, V.; Saint-Pierre, T.D.; Rocha, R.C.; Kai, J.; Xing, Y.; et al. Structural, optical and catalytic properties of ZnO-SiO2 colored powders with the visible light-driven activity. J. Photochem. Photobiol. A 2021, 421, 113532. [Google Scholar] [CrossRef]
- Zabihi, E.; Arab-Bafrani, Z.; Hoseini, S.M.; Mousavi, E.; Babaei, A.; Khalili, M.; Hashemi, M.M.; Javid, N. Fabrication of nano-decorated ZnO-fibrillar chitosan exhibiting a superior performance as a promising replacement for conventional ZnO. Carbohydr. Polym. 2021, 274, 118639. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Imran, M.; Ali, B.; Nawaz, M.; Siddique, M.H.; Al-Kahtani, A.A.; Hussain, K.; Murtaza, B.; Shah, N.S.; Khan, Z.U.H.; et al. Nanocomposites of sedimentary material with ZnO and magnetite for the effective sequestration of arsenic from aqueous systems: Reusability, modeling and kinetics. Environ. Technol. Innov. 2021, 21, 101298. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2022, 431, 134312. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Saad, I.; Khim, J.S.; Ajarem, J.S.; Allam, A.A. Enhanced oxidation of antibiotic residuals (Levofloxacin) using a green composite of ZnO@ polyaniline/bentonite (Zn@ PA/BE) as multifunctional photocatalyst under visible light. Int. J. Environ. Anal. Chem. 2022, 1–21. [Google Scholar] [CrossRef]
- Salam, M.A.; AbuKhadra, M.R.; Mohamed, A.S. Effective oxidation of methyl parathion pesticide in water over recycled glass based-MCM-41 decorated by green Co3O4 nanoparticles. Environ. Pollut. 2020, 259, 113874. [Google Scholar] [CrossRef]
- Basyouny, M.G.; Abukhadra, M.R.; Alkhaledi, K.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Soliman, A.T.A.; Luqman, M. Insight into the catalytic transformation of the waste products of some edible oils (corn oil and palm oil) into biodiesel using MgO/clinoptilolite green nanocomposite. Mol. Catal. 2021, 500, 111340. [Google Scholar] [CrossRef]
- Dardir, F.M.; Mohamed, A.S.; Abukhadra, M.R.; Ahmed, E.A.; Soliman, M.F. Cosmetic and pharmaceutical qualifications of Egyptian bentonite and its suitability as drug carrier for Praziquantel drug. Eur. J. Pharm. Sci. 2018, 8, 320–329. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, T.; Luo, H.; Geng, J.; Yu, W.; Jiang, Z. Adsorption of arsenic by activated charcoal coated zirconium-manganese nanocomposite: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 318–328. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Zhang, A.; Liao, C. Preparation of Fe–Co based MOF-74 and its effective adsorption of arsenic from aqueous solution. J. Environ. Sci. 2019, 80, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As (V), Hg (II), and U (VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Chen, J.; Zhang, X.; Chen, Y. Adsorption of Pb (II) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation: Adsorption capacity, kinetic and isotherm studies. Appl. Surf. Sci. 2014, 293, 160–168. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Sheng, F.; Qing, H. Adsorption characteristics of Copper (II), Zinc (II) and Mercury (II) by four kinds of immobilized fungi residues. Ecotoxicol. Environ. Saf. 2018, 147, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- El-Zeiny, H.M.; Abukhadra, M.R.; Sayed, O.M.; Osman, A.H.; Ahmed, S.A. Insight into novel β-cyclodextrin-grafted-poly (N-vinylcaprolactam) nanogel structures as advanced carriers for 5-fluorouracil: Equilibrium behavior and pharmacokinetic modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124197. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; Reinert, L.; Knani, S.; Duclaux, L.; Lamine, A.B. Experimental and theoretical studies of adsorption of ibuprofen on raw and two chemically modified activated carbons: New physicochemical interpretations. RSC Adv. 2016, 6, 12363–12373. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Bonilla-Petriciolet, A.; Lamine, A.B. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+ and Ni2+ on chicken feathers. J. Mol. Liq. 2020, 319, 114168. [Google Scholar] [CrossRef]
- Sellaoui, L.; Ali, J.; Badawi, M.; Bonilla-Petriciolet, A.; Chen, Z. Understanding the adsorption mechanism of Ag+ and Hg2+ on functionalized layered double hydroxide via statistical physics modeling. Appl. Clay Sci. 2020, 198, 105828. [Google Scholar] [CrossRef]
- Mobarak, M.; Ali, R.A.; Seliem, M.K. Chitosan/activated coal composite as an effective adsorbent for Mn (VII): Modeling and interpretation of physicochemical parameters. Int. J. Biol. Macromol. 2021, 186, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption mechanism of Zn2+, Ni2+, Cd 2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
- Dutta, S.; Manna, K.; Srivastava, S.K.; Gupta, A.K.; Yadav, M.K. Hollow polyaniline Microsphere/Fe3O4 nanocomposite as an effective adsorbent for removal of arsenic from water. Sci. Rep. 2020, 10, 4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Deng, S.; Maimaiti, A.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. As (III) and As (V) adsorption on nanocomposite of hydrated zirconium oxide coated carbon nanotubes. J. Colloid Interface Sci. 2018, 511, 277–284. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Y.; Yang, T.; Yang, Y.; Jiang, Y.; Ma, Z.; Li, X.; Hou, J.; Xi, B.; Liu, H. One-step and acid free synthesis of γ-Fe2O3/SBA-15 for enhanced arsenic removal. Microporous Mesoporous Mater. 2018, 258, 26–32. [Google Scholar] [CrossRef]
- Su, H.; Ye, Z.; Hmidi, N. High-performance iron oxide—Graphene oxide nanocomposite adsorbents for arsenic removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 161–172. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.; Nam, A.; Park, S.J.; Do, T.; Choi, U.S.; Lee, S.H. Arsenic (V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J. Hazard. Mater. 2017, 325, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Chen, J.P. A novel route to the engineering of zirconium immobilized nano-scale carbon for arsenate removal from water. J. Mater. Chem. A 2013, 1, 8636–8644. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Gao, B.; Li, A.; Yang, H. highly selective adsorption of dyes and arsenate from their aqueous mixtures using a silica-sand/cationized-starch composite. Microporous Mesoporous Mater. 2018, 263, 210–219. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.; Liu, Z.Z.; Cao, H.Z.; Hou, G.Y.; Tang, Y.P.; Zheng, G.Q. Highly porous copper ferrite foam: A promising adsorbent for efficient removal of As (III) and As (V) from water. J. Hazard. Mater. 2018, 347, 15–24. [Google Scholar] [CrossRef]









| Kinetic Models | |||||
|---|---|---|---|---|---|
| Material | Model | Parameters | 293 K | 303 K | 313 K |
| BE | Pseudo-First-order | K1 (1/min) | 0.0294 | 0.0335 | 0.0215 |
| Qe(Cal) (mg/g) | 47.7 | 38.7 | 32.58 | ||
| R2 | 0.99 | 0.98 | 0.98 | ||
| X2 | 0.288 | 0.162 | 0.478 | ||
| Pseudo-Second-order | k2 (mg/g min) | 8.71 × 10−4 | 0.0012 | 8.76 × 10−4 | |
| Qe(Cal) (mg/g) | 51.59 | 41.67 | 35.58 | ||
| R2 | 0.96 | 0.97 | 0.92 | ||
| X2 | 0.67 | 0.53 | 0.67 | ||
| PN/BE | Pseudo-First-order | K1 (1/min) | 0.029 | 0.023 | 0.028 |
| Qe(Cal) (mg/g) | 73.5 | 61.29 | 49.42 | ||
| R2 | 0.99 | 0.99 | 0.99 | ||
| X2 | 1.55 | 0.79 | 0.115 | ||
| Pseudo-Second-order | k2 (mg/g min) | 5.75 × 10−4 | 5.41 × 10−4 | 7.2 × 10−4 | |
| Qe(Cal) (mg/g) | 79.26 | 66.42 | 53.9 | ||
| R2 | 0.95 | 0.94 | 0.99 | ||
| X2 | 1.76 | 1.19 | 0.353 | ||
| G.Zn@PN/BE | Pseudo-First-order | K1 (1/min) | 0.0234 | 0.0229 | 0.020 |
| Qe(Cal) (mg/g) | 109.2 | 87.4 | 69.67 | ||
| R2 | 0.99 | 0.99 | 0.99 | ||
| X2 | 0.515 | 0.53 | 0.34 | ||
| Pseudo-Second-order | k2 (mg/g min) | 2.96 × 10−4 | 3.6 × 10−4 | 3.87 × 10−4 | |
| Qe(Cal) (mg/g) | 118.05 | 94.3 | 75.92 | ||
| R2 | 0.97 | 0.97 | 0.97 | ||
| X2 | 1.31 | 1.08 | 0.78 | ||
| Isotderm Models | |||||
| BE | Langmuir model | Qmax (mg/g) | 150.06 | 140.6 | 124.2 |
| b (L/mg) | 0.0018 | 0.003 | 0.0052 | ||
| R2 | 0.84 | 0.87 | 0.88 | ||
| X2 | 3.06 | 3.09 | 2.12 | ||
| Freundlich model | 1/n | 0.595 | 0.728 | 0.843 | |
| kF (mg/g) | 2.58 | 1.075 | 0.449 | ||
| R2 | 0.78 | 0.79 | 0.77 | ||
| X2 | 3.46 | 4.15 | 4.79 | ||
| D-R model | β (mol2/KJ2) | 0.037 | 0.041 | 0.056 | |
| Qm (mg/g) | 75.6 | 68.3 | 55.9 | ||
| R2 | 0.996 | 0.994 | 0.991 | ||
| X2 | 0.057 | 0.109 | 0.182 | ||
| E (KJ/mol) | 3.83 | 3.49 | 2.98 | ||
| PN/BE | Langmuir model | Qmax (mg/g) | 170.4 | 163.7 | 138.9 |
| b (L/mg) | 0.0078 | 0.0061 | 0.0058 | ||
| R2 | 0.94 | 0.95 | 0.94 | ||
| X2 | 0.92 | 0.72 | 0.92 | ||
| Freundlich model | 1/n | 0.49 | 0.54 | 0.55 | |
| kF (mg/g) | 7.41 | 4.83 | 3.78 | ||
| R2 | 0.88 | 0.89 | 0.88 | ||
| X2 | 2.1 | 1.68 | 1.84 | ||
| D-R model | β (mol2/KJ2) | 1.05 | 1.19 | 1.28 | |
| Qm (mg/g) | 115.5 | 101.9 | 85.8 | ||
| R2 | 0.98 | 0.97 | 0.97 | ||
| X2 | 0.22 | 0.47 | 0.43 | ||
| E (KJ/mol) | 0.69 | 0.64 | 0.62 | ||
| G.Zn@PN/BE | Langmuir model | Qmax (mg/g) | 311.8 | 274 | 226.39 |
| b (L/mg) | 0.006 | 0.0056 | 0.005 | ||
| R2 | 0.95 | 0.95 | 0.93 | ||
| X2 | 1.57 | 1.13 | 1.79 | ||
| Freundlich model | 1/n | 0.54 | 0.54 | 0.57 | |
| kF (mg/g) | 9.35 | 7.59 | 5.06 | ||
| R2 | 0.88 | 0.89 | 0.86 | ||
| X2 | 3.7 | 2.79 | 3.48 | ||
| D-R model | β (mol2/KJ2) | 1.22 | 1.29 | 1.48 | |
| Qm (mg/g) | 197.2 | 168.4 | 136.4 | ||
| R2 | 0.96 | 0.93 | 0.96 | ||
| X2 | 1.05 | 1.78 | 1.90 | ||
| E (KJ/mol) | 0.64 | 0.62 | 0.58 | ||
| Steric and Energetic Parameters | ||||||
|---|---|---|---|---|---|---|
| n | Nm (mg/g) | Qsat (mg/g) | C1/2 (mg/L) | ∆E (kJ/mol) | ||
| BE | 293 K | 3.09 | 23.53 | 72.7 | 71.57 | −3.50 |
| 303 K | 3.39 | 19.06 | 64.6 | 80.23 | −3.33 | |
| 313 K | 4.09 | 12.47 | 51 | 81.92 | −3.39 | |
| PN/BE | 293 K | 2.09 | 57.33 | 119.8 | 67.94 | −3.63 |
| 303 K | 1.92 | 56.43 | 108.3 | 78.38 | −3.39 | |
| 313 K | 2.09 | 43.54 | 90.99 | 78.35 | −3.51 | |
| G.Zn@PN/BE | 293 K | 1.94 | 109.86 | 213.1 | 80.52 | −3.22 |
| 303 K | 1.76 | 108.9 | 191.6 | 89.23 | −3.07 | |
| 313 K | 2.13 | 67.88 | 144.5 | 86.13 | −3.26 | |
| Adsorbent | Qmax (mg/g) | Reference |
|---|---|---|
| Goetdite/goetdite P | 34.12 | [16] |
| ZnO/AlSBA-15 | 123.99 | [13] |
| ZrO(OH)2/CNTs | 124.5 | [55] |
| 0.26γ-Fe2O3/SBA-15 | 23.09 | [56] |
| MWCNTs OCH2CO2H | 250 | [14] |
| FeOx-GO | 113 | [57] |
| Chitosan-coated biosorbent | 96.46 | [58] |
| Fe-MWCNTs | 250 | [59] |
| GONRs | 155.6 | [60] |
| MWCNTs | 200 | [14] |
| Iron oxide nanoparticle | 22.91 | [61] |
| Zirconium-nanoscale carbon | 110 | [62] |
| Silica-sand/cationized-starch | 76.63 | [63] |
| GO/CuFe2O4 foam | 124.69 | [64] |
| Green ZnO | 42.3 | This study |
| BE | 72.7 | This study |
| PN/BE | 119.8 | This study |
| G.Zn@PN/BE composite | 213 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Salam, M.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@polynaniline/Bentonite Tripartite Structure (G.Zn@PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. https://doi.org/10.3390/polym14122329
Abdel Salam M, Mokhtar M, Albukhari SM, Baamer DF, Palmisano L, Jaremko M, Abukhadra MR. Synthesis and Characterization of Green ZnO@polynaniline/Bentonite Tripartite Structure (G.Zn@PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers. 2022; 14(12):2329. https://doi.org/10.3390/polym14122329
Chicago/Turabian StyleAbdel Salam, Mohamed, Mohamed Mokhtar, Soha M. Albukhari, Doaa F. Baamer, Leonardo Palmisano, Mariusz Jaremko, and Mostafa R. Abukhadra. 2022. "Synthesis and Characterization of Green ZnO@polynaniline/Bentonite Tripartite Structure (G.Zn@PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties" Polymers 14, no. 12: 2329. https://doi.org/10.3390/polym14122329