1. Introduction
Hydraulic fracturing technology is a frequently used method for fracturing in low permeability rock formations [
1,
2]. These fracturing methods have been implemented in the oil industry for more than 40 years [
3,
4]. So, to induce a fracture and convey the delivered proppant into the fracture, hydraulic fracturing involves injecting a high-pressure fracturing fluid into a reservoir formation [
5,
6]. This process creates a high formation conductivity in near wellbore zones of fractures [
7,
8]. Initially in hydraulic fracturing, polymer fluids such as guar gum [
9,
10] were mainly used as fracturing fluid thickeners [
11,
12,
13,
14,
15,
16,
17]. However, traditional polymer-based fluids produce residues, impairing the formation and lower pore conductivity. In addition, only 30 to 45% of the injected guar-based polymer fluids could return from the well during the flow-back period, as shown in a study conducted by Thomas et al. [
18,
19]. This was caused by the leftover unbroken polymer-based fracturing fluid that obstructed the flow channel [
20,
21,
22]. Moreover, the proppant (sand) can sink to the bottom of the polymer fluids before reaching the fracture tip because of the weak sand suspension capacity. It has been observed that the polymer fluids’ high viscosity can cause fractures to expand in height rather than length [
23,
24,
25].
The fracturing fluid compositions vary based on reservoir rocks and other surrounding factors. The primary function of the fracturing fluid is to fracture the rock and transport proppants in the fracture. So, the fluids should be able to carry and transport sand proppants from surface facilities to the newly created fractures in the subsurface and then break them down so that the proppants can be settled in the fracture gaps. At the same time, the remaining fluid should flow back to the surface. The conventional polymeric fracturing fluids have many issues, such as polymeric and crosslinker residue in the formation, which leads to damage [
15], substantial amount of trapped water, etc. The surfactant-based viscoelastic (SBVE) fluids are deemed capable of eliminating these issues and emerging as an environmentally friendly green technique for fracturing. Since Schlumberger presented the concept of viscoelastic surfactants (VES) or SBVE fluid as a thickening agent for fracturing fluid in 1997 [
26,
27,
28], the viscoelastic behavior of SBVE fluids and the no makeup of the residue after the gel breaks have made them an appealing approach in the oil and gas industry [
29,
30,
31].
Not only good viscosity, but also having a good elasticity enable SBVE fluids to be a perfect alternative candidate to transport proppants [
26]. The worm-like micelles (WLMs) that are responsible for this viscoelastic behavior [
27]. These viscoelastic WLMs are smart self-organized structures that can be applied in a wide range of oil and gas industry operations, such as hydraulic fracturing, emulsions, polymer, surfactant, and foam flooding [
9,
31,
32].
However, these cylindrical micelles are highly susceptible to hydrocarbons. During the completion stage of the hydraulic fracturing operation, the carrier liquid will be destroyed by the influence of the formation hydrocarbon and can be easily removed from the fractures. Consequently, the high permeable path of the fracture will be achieved for the formation fluids to flow. Nonetheless, application of SBVE fluids at high temperature conditions in deep wells is a huge challenge [
33]. The viscous stability of fracturing fluids with respect to the temperature and shear rate changes are key parameters to consider, which determine the proppants’ carrying potential of the fluid [
30,
34,
35].
Therefore, developing improved viscoelastic systems of SBVE fluids using other additives is necessary [
11,
12,
13]. These can provide a high elasticity modulus and viscosity stability at elevated temperatures and moderate filtrate recovery [
14,
15,
16,
36]. In addition, WLMs that react to external stimuli are being researched to control viscoelastic behavior better and understand its applicability under different environments [
37,
38,
39].
Over the last few years, researchers have realized that nanoparticles can improve surfactant-based viscoelastic fluids’ performance. The nanoparticles establish electrostatic bridges to surfactant micelles which modify the microstructural behaviours and rheology of the viscoelastic fluid system. The nanoparticles strengthen the entanglements of worm-like micelles providing increased micellar length and consequently improving rheological characteristics such as viscosity [
30,
40,
41,
42,
43]. The viscoelastic fluid systems consistently showed improved properties and stability under adverse conditions when metal oxide nanoparticles were added [
40,
41,
42,
43].
Cetyltrimethylammonium bromide (CTAB) are quaternary ammonium halides that make spherical micelles after a critical micellar concentration. The micelles of these surfactants grow from spherical to rod-shaped by adding of different counter-ions [
8,
37]. Generally, Halide anions associate with surfactant headgroups moderately with gradual micellar growth. However, with specific anions that associate strongly, such as inorganic and aromatic salt reagent anions (e.g., NO
3− of Sodium Nitrate), the surfactant solutions give a remarkable viscosity increase due to rapid growth in rod-shaped micelles even at low surfactant and salt concentrations [
30,
38,
43].
For instance, Chieng, Z. H., et al. [
44] reported mixing organic acids, citric acid (CA) and maleic acid (MA) at respective molar ratios of (3:1) and (2:1), with long chain cationic surfactant cetyltrimethylammonium bromide (CTAB). This was a novel way to create a CTAB-based VES-fluid solution with the optimum fracture capabilities. Experimental confirmation of the CTAB-based VES-thickening fluid’s viscoelastic behavior at a temperature of 90 °C demonstrated CTAB-CA VES-fluid as desirable thickening fracturing fluid [
44].
In this experimental study, SBVE fluids were synthesized using cationic surfactant CTAB and sodium nitrate (NaNO3) as counter ion salt reagents. Different SBVE fluids are synthesized at a fixed surfactant concentration (0.1 M) and different salt reagent concentrations. This category of fluid is termed type1 fluids. No author has studied this composition of SBVE fluids previously to implement them for hydraulic fracturing purposes. The rheological characteristics: viscosity and shear stress have been analysed using a rotational rheometer by varying shear rates from 1 to 500 sec −1 at 5 sec −1 intervals and different temperatures which is a novel approach to understand the ability of SBVE fluids under fiend-like conditions during fracturing. The authors found a massive drop in viscosity at high temperatures and at high shear rates (HTHS) conditions. Therefore, they investigated other possible ways to improve the SBVE fluid system.
Recently, some studies have been conducted on the application of zinc oxide nanoparticles (ZnO NPs) for drilling fluid compositions under different conditions especially under high temperature conditions. The studies reported that ZnO NPs enhance the fluid properties by providing stable viscosity, less fluid loss, inhibitive nature, and ability to remove H
2S [
45,
46,
47].
Therefore, the authors chose to investigate ZnO NPs with SBVE considering them to be a potential candidate for improvements in rheological characteristics of the synthesized SBVE fluid system. The authors hypothesized that ZnO nanoparticles could improve this fluid’s rheological characteristics under HTHS conditions. Therefore, the next version of fluids is synthesized by implementing nanofluids of zinc oxide (ZnO NPs dispersion in water) were termed type2 fluids. The viscosity plots for all fluids of type1 and type2 categories were analysed at a fixed temperature of 25 °C by varying shear rate values to identify optimum fluids with the highest viscosity values for the entire shear rate range.
The rheology of optimum fluids compared for all shear rate values (1 to 500 Sec−1) at different temperatures of 25 °C, 35 °C, 45 °C, 55 °C, 65 °C and 75 °C respectively to visualize the effect of increasing shear rate conditions at different temperatures. The descriptive plots depict viscosity at each temperature for the range of shear rates which helps to check change in rheological characteristics due to ZnO NPs additives at each case of HTHS.
Further, the authors have developed Herschel–Bulkley fluid models for synthesized viscoelastic fluids systems depending on statistical analysis and correlation parameters identified as consistency, flow index and yield stress on plotted rheometric parameters: viscosity and shear stress values with varying shear rate values at 25 °C temperature conditions. In the last section of this study, the pressure-drop estimation method described using given group equations for laminar flow in a pipe depending on Herschel–Bulkley-model parameters will be identified for optimum fluids in both categories.
2. Materials and Methods
Cetyltrimethylammonium bromide, cetrimonium bromide, hexadecyltrimethylammonium bromide or CTAB is a quaternary ammonium surfactant. It is one of the components of topical antiseptic cetrimide, and its molecular structure is illustrated in
Figure 1. The chemical formula for CTAB is ([(C
16H
33)N(CH
3)
3] Br) with a molecular weight of 364.447 gm/mol. The cationic surfactant CTAB (98% pure) of Loba Chemie Pvt. Ltd. was obtained from Sigma Aldrich. AR grade Sodium Nitrate salt (with a minimum assay of 99%) for anionic nitrate counter ion was obtained from ACS chemicals (Molecular structure in
Figure 1). The nanofluid of ZnO dispersion nanoparticles (<100 nm particle size TEM), 20 wt% in H
2O was obtained from sigma Aldrich.
The aqueous solutions of cationic surfactants such as hexadecyltrimethylammonium bromide (CTAB) form long worm-like micelles (WLMs) upon adding specific salts, strongly binding counter-ions or cosurfactants. The enthalpy of micellization and Gibs free energy for micellization seems to be the lowest for NO
3− [
17,
48] compared with other inorganic anions, as reported by Jiang et al. (2005) [
48], this indirectly indicates the entropy of micellization in CTAB solution. Earlier, K. Kuperkar et al. (2008) [
38] investigated viscoelastic solutions of (WLMs) formed in aqueous solutions of the cationic surfactant CTAB in the presence of the salt reagent NaNO
3. They reported that the addition of NaNO
3 to CTAB micelles leads to a decrease in the surface charge of the ellipsoidal micelles and, thus, an increase in their length occurs. Researchers have also reported that NaNO
3 is a highly effective inorganic electrolyte to induce worm-like micelle (WLMs) formation and branching in the micellar solution of Cetyltrimethylammonium bromide (CTAB) [
38].
2.1. Preparation of Type1 Fluids without Nanoparticle Additives
The surfactant solution was prepared with a fixed CTAB concentration of 0.1 M in demineralized water, and different viscoelastic fluids were prepared by varying salt concentrations from 0.2 M to 2.0 M.
The transparent surfactant solution (d) was prepared by ultrasonication bathing (c) of white solution of demineralized solvent water (a) and solute cetrimonium bromide (b), as shown in
Figure 2. Then the inorganic sodium nitrate salt reagent was added, and the prepared solution (e) was mixed by heating and stirring (f) using a magnetic stirrer. The prepared fluid goes through an ultrasonic bath (g) which removes air bubbles in the fluid, and a homogeneous viscoelastic surfactant fluid (h) fluid is prepared.
2.2. Preparation of Type2 Fluid with Nanoparticle Additives
Initially, the 1 wt% ZnO NP dispersion nanofluid solution was prepared using demineralized water and adding 20 wt% ZnO nanoparticle dispersion fluid (in water). The surfactant solution was prepared with a fixed 0.1 M CTAB concentration and nanofluid solution. Then different viscoelastic fluids were prepared by varying salt concentrations from 0.2 M to 2 M.
Initially, a homogenous 1% weight of ZnO nanoparticle dispersion in water (1 wt% ZnO nanofluid) (a) was prepared using demineralized water and 20% weight ZnO nanoparticle dispersion in water obtained from Sigma Aldrich(Gujarat, India). The prepared fluid goes through an ultrasonic bath (h) which removes air bubbles in the fluid, and a homogeneous viscoelastic surfactant fluid (i) is prepared. The slightly white surfactant solution (e) was prepared by ultrasonication bathing (d) of white solution of solvent nanofluid (b) and solute Cetrimonium bromide (c). Then the inorganic sodium nitrate salt reagent was added, and the prepared solution (f) was mixed by heating and stirring (g) using a magnetic stirrer, as shown in
Figure 3.
4. Fluid Modelling and Pressure Drop during Laminar Flow in Pipeline
Winslow Herschel and Ronald Bulkley’s model introduced the model of non-Newtonian fluids in 1926, in which the strain experienced by the fluid is related to the stress in a complicated and non-linear way. The relationship is characterized by three parameters which are the consistency
k, the flow index
n, and the yield shear stress
τ0. The flow index measures the degree to which the fluid is shear-thinning or shear-thickening, and the consistency is a simple constant of proportionality [
50,
51]. The yield stress quantifies the amount of stress the fluid may experience before it yields or deforms and begins to flow.
We can estimate consistency value k and flow index value n by analyzing statistical regression functions of all viscoelastic fluids of type1 and type2.
The constitutive equations of the Herschel–Bulkley model after the yield stress have been reached and can be written as follows (Equations (1) and (2)) [
52,
53,
54]
Here 𝜏 is shear stress values in Pa, 𝜏0 is the yield stress value in Pa, ϓ is shear rate values in Sec−1, k is fluid consistency, n is flow index and η is viscosity in Pa-Second or Centipoise.
Therefore, the constitutive equation of the Herschel–Bulkley model after the yield stress has been reached for optimum fluids can also be estimated.
The Herschel–Bulkley model equation (after yield stress has been reached) for the optimum fluid of type1 category fluids without nanoparticle additives, fluid with 0.8 M NaNO
3 concentration can be expressed as below, where the values have been taken from
Table 3 and
Table 5.
Similarly, the Herschel–Bulkley model equation (after yield stress has been reached) for the optimum fluid of type2 category fluids with nanoparticle additives, fluid with 0.6 M NaNO
3 concentration can be expressed as below. The values have been taken from
Table 4 and
Table 6.
Chilton and Stains represented a set of equations (Equations (7)–(11)) to calculate the pressure drop for laminar flow for such fluids [
55]. The equations require an iterative method to extract the pressure drop, as it is present on both sides of the equation [
52,
55].
Here P is the Pressure Drop in Pa, L is the pipe length in meters, and D is the diameter of the pipe in meters.
Therefore, as suggested by Chilton and Stains, the pressure drop during laminar flow in a pipe can be estimated for both optimum fluids of type1 and type2 groups using factors calculated
a,
b, and
c in
Table 7 and implementing an iterative method as suggested by the authors [
55].
5. Discussion
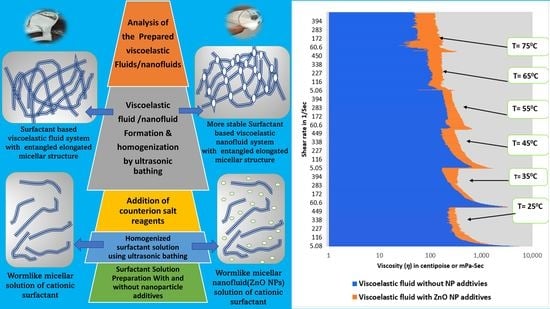
Micellar solution of surfactants having wormlike micelles or cylindrical micelles changes to viscoelastic fluids with good rheological characteristics under certain conditions due to entanglements in micelles. These viscoelastic systems are sensitive to changes in conditions. So, these viscoelastic systems are not able to maintain rheology under high temperature and high shear rate (HTHS) conditions. The nanoparticle additives can assist to maintaining or improving their rheology even at HTHS conditions.
In this study, initially, a micellar solution of cationic surfactant of cetrimonium bromide or CTAB was prepared which had long cylindrical or worm-like micelles. The micellar solution formed a highly viscous, viscoelastic fluid system in the presence of counterion sodium nitrate salt reagents due to entanglements in long WLMs of CTAB. The rheology was analyzed using a rotational rheometer with varying temperature and shear rates. The represented rheology of the fluids was found to be high enough that the fluids can be implemented successfully on the field for hydraulic fracturing operations (see in the
Supplementary Materials). These SBVE fluids leave no residual of polymers or crosslinkers in the formations near the fractured area. Therefore, these SBVE fluids have the ability to avoid formation damage near fractured area.
The rheology of the fluids changes with varying concentrations of the salt reagent. The viscosities show high values up to certain concentrations of the counter ion salt reagent for both fluid categories with and without ZnO nanoparticles additives: type1 and type2 fluids. Beyond which the fluids represent lesser viscosity values when increasing the salt concentration, which is same for both the fluid categories. Hence, the optimum fluid concentrations have been identified as 0.8 M NaNO
3 salt concentration for type1 fluids and 0.6 M NaNO
3 for type2 fluids which show the highest viscosity for all shear rate and temperature conditions. The rheological characteristics have been analyzed using a rotation rheometer. The viscosity values for both types of fluids decrease with increasing the shear rates and temperature conditions. However, the authors hypothesized that ZnO NPs would improve the rheology of type1 fluids by supporting entangled WLMs structures, which is proven true. The average viscosity comparison of optimum fluids at 25 °C and different shear rate ranges has been illustrated in
Figure 15.
The viscosity values of the type2 optimum fluid (0.6 M salt reagent concentration) with ZnO nanoparticles additives are higher when compared to type1 (without nano additives) fluids of 0.6 and 0.8 (optimum) salt concentration for entire range of shear rates (1 to 500 Sec
−1) at all temperature conditions: 25 °C, 35 °C, 45 °C, 55 °C, 65 °C and 75 °C respectively as illustrated in
Figure 10 and
Figure 11. The authors analyzed the rheometric data to get correlations and fluid and pressure drop equation models.
The effect of temperature on the rheology of synthesized optimum SBVE fluids is illustrated in
Figure 6 and
Figure 9, which show that viscosity values at any constant shear rate decreased gradually with an increase in temperature of type1 (without nano additives) optimum fluid system while it remains similar up to 55 °C in the case of type2 (with ZnO nano additives) optimum fluid and shows lesser viscosities at 65 °C and 75 °C. Therefore, the ZnO nanoparticle additives have the ability to maintain the rheology of the SBVE system with increasing temperature conditions up to 55 °C.
The statistical analysis and correlations of rheological characteristics with varying shear rates at 25 °C proves that the fluid follows Herschel–Bulkley fluid models. Flow index, consistency and yield stress were identified to characterize the fluids. Subsequently, method and equation models are suggested for the estimation of pressure drop during laminar flow in a pipe depending on the identified characteristic parameters of Herschel–Bulkley models. These models and methods will help to understand the behaviour of SBVE fluids during their on-field implementations for hydraulic fracturing purposes. However, these SBVE fluid systems should be investigated more profoundly before their on-field implementations considering other aspects of hydraulic fracturing operations, such as how the fluids behave with different rock mineralogy, what type of oil and gas formations are best suited for these fluids and what other compositions (such as breakers, friction reducers etc.) can be added to them to cover remaining important technical aspects for a successful on-field applications during hydraulic fracturing operations.