Synthesis and Characterization of Cyclodextrin-Based Polyhemiaminal Composites with Enhanced Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
2.3.1. Synthesis of ODA-PHA
2.3.2. Synthesis of CD/ODA-PHA
2.4. NMR Spectroscopic Analysis
2.5. FTIR Analysis
2.6. Raman Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Raman Analysis
3.3. Powder XRD Analysis
3.4. Hemiaminal Core Studies Using NMR Spectroscopy
3.5. TGA
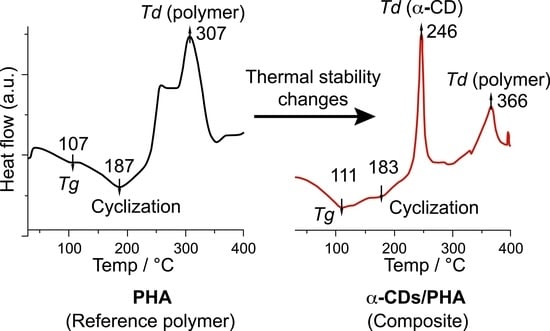
3.6. DSC
3.7. Elemental Analysis
3.8. FE-SEM
3.9. AFM
3.10. Solubility Assay of ODA-PHA and CD/ODA-PHA in NMP/LiBr
3.11. Diamine Recovery Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- García, J.M.; Jones, G.O.; Virwani, K.; McCloskey, B.D.; Boday, D.J.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M.S.; et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science 2014, 344, 732–735. [Google Scholar]
- Fox, C.H.; ter Hurrne, G.M.; Wojtecki, R.J.; Jones, G.O.; Horn, H.W.; Meijer, E.W.; Frank, C.W.; Hedrick, J.L.; García, J.M. Supramolecular motifs in dynamic covalent PEG-hemiaminal organogels. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hiller, M.; Evsyukov, S.E. Laser-engravable hexahydrotriazine polymer networks. Mater. Res. Innov. 2002, 6, 179–184. [Google Scholar] [CrossRef]
- Lippert, T.; Dickinson, J.T. Chemical and spectroscopic aspects of polymer ablation: Special features and novel directions. Chem. Rev. 2003, 103, 453–485. [Google Scholar] [CrossRef]
- Lippert, T.; Hauer, M.; Phipps, C.R.; Wokaun, A. Fundamentals and applications of polymers designed for laser ablation. Appl. Phys. A Mater. Sci. Process. 2003, 77, 259–264. [Google Scholar] [CrossRef]
- Kaminker, R.; Callaway, E.B.; Dolinski, N.D.; Barbon, S.M.; Shibata, M.; Wang, H.; Hu, J.; Hawker, C.J. Solvent-free synthesis of high-performance polyhexahydrotriazine (PHT) thermosets. Chem. Mater. 2018, 30, 8352–8358. [Google Scholar] [CrossRef]
- You, S.; Ma, S.; Dai, J.; Jia, Z.; Liu, X.; Zhu, J. Hexahydro-s-triazine: A trial for acid-degradable epoxy resins with high performance. ACS Sustain. Chem. Eng. 2017, 5, 4683–4689. [Google Scholar] [CrossRef]
- Kappes, R.S.; Schönfeld, F.; Li, C.; Golriz, A.A.; Nagel, M.; Lippert, T.; Butt, H.J.; Gutmann, J.S. A study of photothermal laser ablation of various polymers on microsecond time scales. Springerplus 2014, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lippert, B.T.; Yabe, A.; Wokaun, A. Laser ablation of doped polymer systems. Adv. Mater. 1997, 9, 105–119. [Google Scholar] [CrossRef]
- Jones, G.O.; García, J.M.; Horn, H.W.; Hedrick, J.L. Computational and experimental studies on the mechanism of formation of poly(hexahydrotriazine)s and poly(hemiaminal)s from the reactions of amines with formaldehyde. Org. Lett. 2014, 16, 5502–5505. [Google Scholar] [CrossRef]
- Fevre, M.; Wojtecki, R.J.; Hedrick, J.; García, J.M.; Hedrick, J.L. Tailorable thermal properties through reactive blending using orthogonal chemistries and layer-by-layer deposition of poly(1,3,5-hexahydro-1,3,5-triazine) networks. Adv. Funct. Mater. 2016, 26, 5560–5568. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jia, X.; Yang, J.; Li, Y.; Song, H. Interfacial modification and tribological properties of carbon fiber grafted by TiO2 nanorods reinforced novel depolymerized thermosetting composites. Compos. Part A Appl. Sci. Manuf. 2020, 133, 1–11. [Google Scholar] [CrossRef]
- Xu, N.; Kim, S.; Liu, Y.; Adraro, Y.A.; Li, Z.; Hu, Z.; Liu, L.; Hu, Z.; Huang, Y. Facile preparation of rapidly recyclable tough thermosetting composites via crosslinking structure regulation. Polymer 2020, 189, 1–7. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stab. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.H.; Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef]
- Takata, T.; Aoki, D. Topology-transformable polymers: Linear-branched polymer structural transformation via the mechanical linking of polymer chains. Polym. J. 2018, 50, 127–147. [Google Scholar] [CrossRef]
- Wenz, G.; Steinbrunn, M.B.; Landfester, K. Solid state polycondensation within cyclodextrin channels leading to water soluble polyamide rotaxanes. Tetrahedron 1997, 53, 15575–15592. [Google Scholar] [CrossRef]
- Yang, J.Y.; Te Jung, B.; Suh, D.H. A simple attempt to change the solubility of polyimide by physical inclusion with β-cyclodextrin and its derivatives. Polymer 2001, 42, 8349–8354. [Google Scholar] [CrossRef]
- Sarker, F.; Karim, N.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Potluri, P. Ultrahigh Performance of Nanoengineered Graphene-Based Natural Jute Fiber Composites. ACS Appl. Mater. Interfaces 2019, 11, 21166–21176. [Google Scholar] [CrossRef]
- Sarker, F.; Potluri, P.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Karim, N. High-performance graphene-based natural fiber composites. ACS Appl. Mater. Interfaces 2018, 10, 34502–34512. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Ito, K. New solvent for polyrotaxane. I. Dimethylacetamide/lithium chloride (DMAc/LiCl) system for modification of polyrotaxane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 532–538. [Google Scholar] [CrossRef]
- Bouzit, H.; Stiti, M.; Abdaoui, M. Spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin with N-[(4-sulfonamidophenyl) ethyl]-5-(1, 2-dithiolan-3-yl) pentanamide. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 121–134. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high-performance thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Tang, X.Z.; Li, W.; Yu, Z.Z.; Rafiee, M.A.; Rafiee, J.; Yavari, F.; Koratkar, N. Enhanced thermal stability in graphene oxide covalently functionalized with 2-amino-4, 6-didodecylamino-1, 3,5-triazine. Carbon 2011, 49, 1258–1265. [Google Scholar] [CrossRef]
- Sivakumar, K.; Parinamachivayam, G.; Murali Krishnan, M.; Chakravarty, S.; Bharathi, A. Preparation, characterization and molecular modeling studies of the beta-cyclodextrin inclusion complex with benzoguanamine and its analytical application as chemosensor for the selective sensing of Ce4+. Spectrochim. Acta Part A 2018, 200, 212–225. [Google Scholar] [CrossRef]
- Rusa, C.C.; Luca, C.; Tonelli, A.E. Polymer-cyclodextrin inclusion compounds: Toward new aspects of their inclusion mechanism. Macromolecules 2001, 34, 1318–1322. [Google Scholar] [CrossRef]
- Ghiorghiasa, R.; Petrovici, A.R.; Rosca, I.; Miron, L. Inclusion complex of thiotriazinone with α-cyclodextrin-Raman spectroscopy, DSC, preliminary antimicrobial and antifungal study. Dig. J. Nanomater. Bios. 2016, 11, 235–241. [Google Scholar]
- Sano, H.; Ichi, T.; Kumashiro, Y.; Kontani, K.; Kuze, T.; Mizutani, G.; Ooya, T.; Yui, N. Raman scattering study of water clusters around polyrotaxane and pseudopolyrotaxane supramolecular assemblies. Spectrochim. Acta Part A 2003, 59, 285–289. [Google Scholar] [CrossRef]
- Thurein, S.M.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical properties of β-cyclodextrin solutions and precipitates prepared from injectable vehicles. Asian J. Pharm. Sci. 2018, 5, 438–449. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.J.; Gao, P.; Jiang, L.; Li, S.; Feng, Z.G. Distinguishing channel-type crystal structure from dispersed structure in β-cyclodextrin based polyrotaxanes via FTIR spectroscopy. Front. Mater. Sci. 2011, 5, 329–334. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, Y.S.; Lin, H.T.; Wang, C.F. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Haynes, A.; Halpert, P.; Levine, M. Colorimetric Detection of Aliphatic Alcohols in β-Cyclodextrin Solutions. ACS Omega 2019, 4, 18361–18369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.L.; Zhang, H.Y.; Li, X.Y.; Liang, P.; Zhang, X.Z.; Xu, J.J. Supramolecular polypseudorotaxane with conjugated polyazomethine prepared directly from two inclusion complexes of β-cyclodextrin with tolidine and phthaldehyde. Macromolecules 2004, 37, 6362–6369. [Google Scholar] [CrossRef]









| ODA:CD Molar Ratio | PFA/Formalin eq. vs. ODA 1 | Sonication Time (h) | Reaction Time (h) | Isolated Yield (wt.%) |
|---|---|---|---|---|
| 1:0 (ODA) | 4.5 (PFA) | – | 24 | 85–90 |
| 1:0.5 (α-CD) | 4.5 (PFA) | 1 | 8–10 | 85–90 |
| 1:0.5 (α-CD) | 4.5 (formalin) | 1 | 2–3 | 85–90 |
| 1:0.5 (β-CD) | 4.5 (formalin) | 1 | 3–4 | 80–85 |
| 1:0.5 (γ-CD) | 4.5 (formalin) | 1 | 20–24 | <75 |
| Compound | Td (°C) 1 | Char Yield (wt.%) 2 | Experimental C/H/N/O Contents (wt.%) 3 | Calcd. C/H/N/O Contents (wt.%) and Formulas 4 |
|---|---|---|---|---|
| ODA-PHA | 293 | 65.5 | 65.9/6.1/11.4/16.6 | 66.2/6.6/11.9/15.3 (C52H62N8O9) |
| α-CD/ODA-PHA | 365 | 39.8 | 54.0/6.7/7.1/32.2 | 55.2/6.4/5.9/32.6 (C88H122N8O39) |
| β-CD/ODA-PHA | 366 | 42.9 | 55.3/6.8/6.7/31.2 | 55.3/6.3/5.5/32.9 (C94H128N8O42) |
| γ-CD/ODA-PHA | 364 | 41.1 | 54.7/6.9/6.2/32.2 | 54.5/6.3/5.1/34.1 (C100H138N8O47) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabedul, H.M.; Toda, M.; Mase, N. Synthesis and Characterization of Cyclodextrin-Based Polyhemiaminal Composites with Enhanced Thermal Stability. Polymers 2022, 14, 1562. https://doi.org/10.3390/polym14081562
Jabedul HM, Toda M, Mase N. Synthesis and Characterization of Cyclodextrin-Based Polyhemiaminal Composites with Enhanced Thermal Stability. Polymers. 2022; 14(8):1562. https://doi.org/10.3390/polym14081562
Chicago/Turabian StyleJabedul, Hoque Mohammed, Mitsuo Toda, and Nobuyuki Mase. 2022. "Synthesis and Characterization of Cyclodextrin-Based Polyhemiaminal Composites with Enhanced Thermal Stability" Polymers 14, no. 8: 1562. https://doi.org/10.3390/polym14081562