Hydrogen Chloride and Sulfur Dioxide Gas Evolutions from the Reaction between Mg Sulfate and NaCl: Implications for the Sample Analysis at the Mars Instrument in Gale Crater, Mars
Abstract
:1. Introduction
1.1. Background
1.1.1. SAM Instrument Overview
1.1.2. Gale Crater Samples
1.1.3. Detection of NaCl and Mg Sulfate in Gale Crater Samples
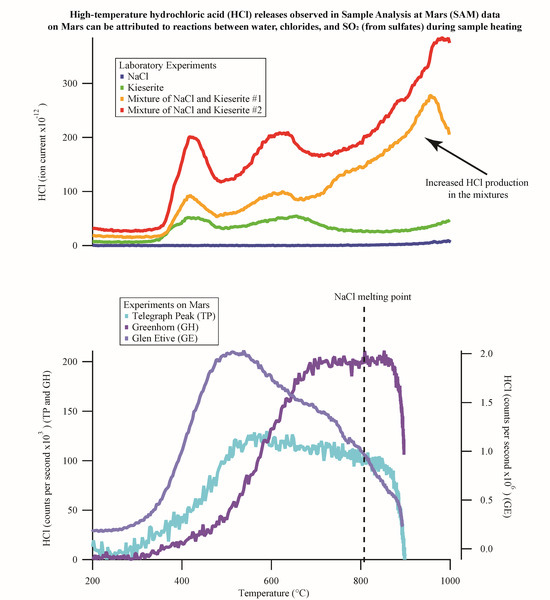
1.1.4. HCl and SO2 Detected by SAM-EGA
HCl
SO2
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Laboratory Thermal and Evolved Gas Analysis
2.3. Laboratory-Powered X-ray Diffraction Analysis
3. Results
3.1. NaCl
3.2. Kieserite
3.3. Equal Molar Mixture
3.4. Mars-like Mixture
4. Discussion
4.1. Comparison with Gale Crater Samples
4.2. Implication of Detecting Mg Sulfates and Chlorides in Gale Crater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milliken, R.E.; Grotzinger, J.P.; Thomson, B.J. Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Powell, K.E.; Arvidson, R.E.; Edwards, C.S. Spectral Properties of the Layered Sulfate-Bearing Unit in Mount Sharp, Gale Crater, Mars. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2023. [Google Scholar]
- Sheppard, R.Y.; Milliken, R.E.; Robertson, K.M. Presence of clay minerals can obscure spectral evidence of Mg sulfates: Implications for orbital observations of Mars. Icarus 2022, 383, 115083. [Google Scholar] [CrossRef]
- Fraeman, A.A.; Ehlmann, B.L.; Arvidson, R.E.; Edwards, C.S.; Grotzinger, J.P.; Milliken, R.E.; Quinn, D.P.; Rice, M.S. The stratigraphy and evolution of lower Mount Sharp from spectral, morphological, and thermophysical orbital data sets. J. Geophys. Res. Planet. 2016, 121, 1713–1736. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dietrich, W.E.; Lewis, K.W.; Kite, E.; Mondro, C.A.; Schieber, J.; Weitz, C.; Bryk, A.B.; Edgar, L.; Fedo, C.; et al. “High” but not so dry on Aeolis Mons: Transient lake systems in Hesperian deserts in Gale Crater. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2023. [Google Scholar]
- Vaniman, D.; Bish, D.; Chipera, S.J.; Fialips, C.I.; Carey, J.W.; Feldman, W.C. Magnesium sulphate salts and the history of water on Mars. Nature 2004, 431, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Chipera, S.J.; Vaniman, D. Experimental stability of magnesium sulfate hydrates that may be present on Mars. Geochim. Cosmochim. Acta 2007, 71, 241–250. [Google Scholar] [CrossRef]
- Thomas, N.H.; Ehlmann, B.L.; Meslin, P.Y.; Rapin, W.; Anderson, D.E.; Rivera-Hernandez, F.; Forni, O.; Schroeder, S.; Cousin, A.; Mangold, N.; et al. Mars Science Laboratory Observations of Chloride Salts in Gale Crater, Mars. Geophys. Res. Lett. 2019, 46, 10754–10763. [Google Scholar] [CrossRef] [PubMed]
- Tosca, N.J.; McLennan, D.; Lindsley, D.H.; Schoonen, M.A. Acid-sulfate weathering of synthetic Martian basalt: The acid fog model revisited. J. Geophys. Res. Planets 2004, 109. [Google Scholar] [CrossRef]
- Clark, J.V.; Sutter, B.; McAdam, A.C.; Rampe, E.B.; Archer, P.D.; Ming, D.W.; Navarro-Gonzalez, R.; Mahaffy, P.; Lapen, T.J. High-Temperature HCl Evolutions From Mixtures of Perchlorates and Chlorides With Water-Bearing Phases: Implications for the SAM Instrument in Gale Crater, Mars. J. Geophys. Res. Planet. 2020, 125, e2019JE006173. [Google Scholar] [CrossRef]
- McAdam, A.; Knudson, C.; Sutter, B.; Franz, H.; Archer, P.D.; Eigenbrode, J.; Ming, D.W.; Morris, R.; Hurowitz, J.A.; Mahaffy, P. Reactions involving calcium and magnesium sulfates as potential sources of sulfur dioxide during MSL SAM evolved gas analyses. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2023. [Google Scholar]
- Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Conrad, P.G.; Coll, P.; Atreya, S.K.; Arvey, R.; Barciniak, M.; Benna, M.; Bleacher, L.; et al. The Sample Analysis at Mars Investigation and Instrument Suite. Space Sci. Rev. 2012, 170, 401–478. [Google Scholar] [CrossRef]
- Sutter, B.; McAdam, A.C.; Mahaffy, P.R.; Ming, D.W.; Edgett, K.S.; Rampe, E.B.; Eigenbrode, J.L.; Franz, H.B.; Freissinet, C.; Grotzinger, J.P.; et al. Evolved gas analyses of sedimentary rocks and eolian sediment in Gale Crater, Mars: Results of the Curiosity rover’s sample analysis at Mars instrument from Yellowknife Bay to the Namib Dune. J. Geophys. Res. Planet. 2017, 122, 2574–2609. [Google Scholar] [CrossRef]
- Webster, C.R.; Mahaffy, P.R.; Atreya, S.K.; Flesch, G.J.; Farley, K.A. Measurements of Mars Methane at Gale Crater by the SAM Tunable Laser Spectrometer on the Curiosity Rover. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2023. [Google Scholar]
- Freissinet, C.; Glavin, D.P.; Mahaffy, P.R.; Miller, K.E.; Eigenbrode, J.L.; Summons, R.E.; Brunner, A.E.; Buch, A.; Szopa, C.; Archer, P.D.; et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J. Geophys. Res. Planet. 2015, 120, 495–514. [Google Scholar] [CrossRef]
- Achilles, C.N.; Downs, R.T.; Ming, D.W.; Rampe, E.B.; Morris, R.V.; Treiman, A.H.; Morrison, S.M.; Blake, D.F.; Vaniman, D.T.; Ewing, R.C.; et al. Mineralogy of an active eolian sediment from the Namib dune, Gale crater, Mars. J. Geophys. Res. Planet. 2017, 122, 2344–2361. [Google Scholar] [CrossRef]
- Rampe, E.B.; Bristow, T.F.; Morris, R.V.; Morrison, S.M.; Achilles, C.N.; Ming, D.W.; Vaniman, D.T.; Blake, D.F. Mineralogy of Vera Rubin Ridge from the Mars Science Laboratory CheMin Instrument. J. Geophys. Res. Planets 2020, 125, e2019JE006306. [Google Scholar] [CrossRef]
- Rampe, E.B.; Lapotre, M.G.A.; Bristow, T.F.; Arvidson, R.E.; Morris, R.V.; Achilles, C.N.; Weitz, C.; Blake, D.F.; Ming, D.W.; Morrison, S.M.; et al. Sand Mineralogy Within the Bagnold Dunes, Gale Crater, as Observed In Situ and From Orbit. Geophys. Res. Lett. 2018, 45, 9488–9497. [Google Scholar] [CrossRef]
- Achilles, C.N.; Rampe, E.B.; Downs, R.T.; Bristow, T.F.; Ming, D.W.; Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Yen, A.S.; McAdam, A.C.; et al. Evidence for Multiple Diagenetic Episodes in Ancient Fluvial-Lacustrine Sedimentary Rocks in Gale Crater, Mars. J. Geophys. Res. Planet. 2020, 125, e2019JE006295. [Google Scholar] [CrossRef]
- Fedo, C.M.; Bryk, A.B.; Edgar, L.A.; Bennett, K.A.; Fox, V.K.; Dietrich, W.E.; Banham, S.G.; Gupta, S.; Stack, K.M.; Williams, R.M.E.; et al. Geology and stratigraphic correlation of the Murray and Carolyn Shoemaker formations across the Glen Torridon region, Gale crater, Mars. J. Geophys. Res. Planets 2022, 127, e2022JE007408. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Bish, D.L.; Ming, D.W.; Bristow, T.F.; Morris, R.V.; Blake, D.F.; Chipera, S.J.; Morrison, S.M.; Treiman, A.H.; Rampe, E.B.; et al. Mineralogy of a Mudstone at Yellowknife Bay, Gale Crater, Mars. Science 2014, 343, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Treiman, A.H.; Bish, D.L.; Vaniman, D.T.; Chipera, S.J.; Blake, D.F.; Ming, D.W.; Morris, R.V.; Bristow, T.F.; Morrison, S.M.; Baker, M.B.; et al. Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin X-ray diffraction of the Windjana sample (Kimberley area, Gale Crater). J. Geophys. Res. Planet. 2016, 121, 75–106. [Google Scholar] [CrossRef] [PubMed]
- Rampe, E.B.; Ming, D.W.; Blake, D.F.; Bristow, T.F.; Chipera, S.J.; Grotzinger, J.P.; Morris, R.V.; Morrison, S.M.; Vaniman, D.T.; Yen, A.S.; et al. Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S.; et al. Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Thorpe, M.T.; Bristow, T.F.; Rampe, E.B.; Tosca, N.J.; Grotzinger, J.; Bennett, K.A.; Achilles, C.N.; Blake, D.F.; Chipera, S.J.; Downs, G.W.; et al. Mars Science Laboratory CheMin Data From the Glen Torridon Region and the Significance of Lake-Groundwater Interactions in Interpreting Mineralogy and Sedimentary History. J. Geophys. Res. Planets 2022, 127, e2021JE007099. [Google Scholar] [CrossRef]
- Berger, J.A.; Gellert, R.; Boyd, N.I.; King, P.L.; McCraig, M.A.; O’Connell-Cooper, C.D.; Schmidt, M.E.; Spray, J.G.; Thompson, L.M.; VanBommel, S.J.; et al. Elemental Composition and Chemical Evolution of Geologic Materials in Gale Crater, Mars: APXS Results From Bradbury Landing to the Vera Rubin Ridge. J. Geophys. Res. Planets 2020, 125, e2020JE006536. [Google Scholar] [CrossRef]
- O’Connell-Cooper, C.D.; Thompson, L.M.; Spray, J.G.; Berger, J.A.; Gellert, R.; McCraig, M.; VanBommel, S.J.; Yen, A. Statistical Analysis of APXS-Derived Chemistry of the Clay-Bearing Glen Torridon Region and Mount Sharp Group, Gale Crater, Mars. J. Geophys. Res. Planets 2022, 127, e2021JE007177. [Google Scholar] [CrossRef]
- Rapin, W.; Ehlmann, B.L.; Dromart, G.; Schieber, J.; Thomas, N.H.; Fischer, W.W.; Fox, V.K.; Stein, N.T.; Nachon, M.; Clark, B.C.; et al. An interval of high salinity in ancient Gale crater lake on Mars. Nat. Geosci. 2019, 12, 889–895. [Google Scholar] [CrossRef]
- Fraeman, A.A.; Edgar, L.A.; Rampe, E.B.; Thompson, L.M.; Frydenvang, J.; Fedo, C.M.; Catalano, J.G.; Dietrich, W.E.; Gabriel, T.S.; Vasavada, A.R.; et al. Evidence for a Diagenetic Origin of Vera Rubin Ridge, Gale Crater, Mars: Summary and Synthesis of Curiosity’s Exploration Campaign. J. Geophys. Res. Planet. 2020, 125, e2020JE006527. [Google Scholar] [CrossRef]
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J. Geophys. Res. Planet. 2013, 118, 1955–1973. [Google Scholar] [CrossRef]
- Leshin, L.A.; Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Coll, P.; Conrad, P.G.; Archer, P.D.; Atreya, S.K.; Brunner, A.E.; Buch, A.; et al. Volatile, Isotope, and Organic Analysis of Martian Fines with the Mars Curiosity Rover. Science 2013, 341, 1238937. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.W.; Archer, P.D.; Glavin, D.P.; Eigenbrode, J.L.; Franz, H.B.; Sutter, B.; Brunner, A.E.; Stern, J.C.; Freissinet, C.; McAdam, A.C.; et al. Volatile and Organic Compositions of Sedimentary Rocks in Yellowknife Bay, Gale Crater, Mars. Science 2014, 343, 1245267. [Google Scholar] [CrossRef]
- McAdam, A.C.; Sutter, B.; Archer, P.D.; Franz, H.B.; Wong, G.M.; Lewis, J.M.T.; Clark, J.V.; Millan, M.; Williams, A.J.; Eigenbrode, J.L.; et al. Evolved gas analyses of sedimentary rocks from the Glen Torridon Clay-Bearing Unit, Gale crater, Mars: Results from the Mars Science Laboratory Sample Analysis at Mars Instrument Suite. J. Geophys. Res. Planet. 2022, 127, e2022JE007179. [Google Scholar] [CrossRef]
- Sutter, B.; McAdam, A.C.; Wong, G.M.; Clark, J.V.; Archer, P.D.; Franz, H.B.; Gasda, P.J.; Ming, D.W.; Yen, A.; Lewis, J.M.T.; et al. Constraining Alteration Processes Along the Siccar Point Group Unconformity, Gale Crater, Mars: Results From the Sample Analysis at Mars Instrument. J. Geophys. Res. Planets 2022, 127, e2022JE007387. [Google Scholar] [CrossRef]
- McAdam, A.C.; Sutter, B.; Archer, P.D.; Franz, H.B.; Wong, G.M.; Lewis, J.M.T.; Eigenbrode, J.L.; Stern, J.C.; Knudson, C.A.; Clark, J.V.; et al. Constraints on the Mineralogy and Geochemistry of Vera Rubin Ridge, Gale Crater, Mars, From Mars Science Laboratory Sample Analysis at Mars Evolved Gas Analyses. J. Geophys. Res. Planet. 2020, 125, e2019JE006309. [Google Scholar] [CrossRef]
- Uchida, S.; Kamo, H. Reaction kinetics of formation of HCl in municipal refuse incinerators. Ind. Eng. Chem. Proc. Dd 1983, 22, 144–149. [Google Scholar] [CrossRef]
- Stern, J.C.; Sutter, B.; Archer, P.D.; Eigenbrode, J.L.; McAdam, A.C.; Franz, H.B.; Knudson, C.; Ming, D.W.; Wong, G.; Freissinet, C.; et al. Major Volatiles Evolved From Eolian Materials in Gale Crater. Geophys. Res. Lett. 2018, 45, 10240–10248. [Google Scholar] [CrossRef]
- Rapin, W.; Chauvire, B.; Gabriel, S.J.; McAdam, A.; Ehlmann, B.L.; Hardgrove, C.; Meslin, P.-Y.; Rondeau, B.; Dehouck, E.; Franz, H.; et al. In Situ Analysis of Opal in Gale Crater, Mars. J. Geophys. Res. Planets 2018, 123, 1955–1972. [Google Scholar] [CrossRef]
- Zhang, P.; Cheng, J.; Jin, Y.; An, X. Evaluation of thermal physical properties of molten nitrate salts with low melting temperature. Sol. Energy Mater. Sol. Cells 2018, 176, 36–41. [Google Scholar] [CrossRef]
- Olivares, R.; Chen, C.; Wright, S. The Thermal Stability of Molten Lithium–Sodium–Potassium Carbonate and the Influence of Additives on the Melting Point. J. Sol. Energy Eng. 2012, 134, 041002. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kritz, G.S. Introduction to Organic Laboratory Techniques: A Contemporary Approach; Saunders College Publishing: Philadephia, PA, USA, 1982. [Google Scholar]
- Henriksson, M.; Warnqvist, B. Kinetics of Formation of Hcl(G) by the Reaction between Nacl(S) and So2, O2, and H2O(G). Ind. Eng. Chem. Proc. Dd 1979, 18, 249–254. [Google Scholar] [CrossRef]
- Robinson, H. Improvements in the Manufacture of Sulphate of Soda. Patent No. 149,859, 21 April 1872. [Google Scholar]
- Scheidema, M.N.; Taskinen, P. Decomposition Thermodynamics of Magnesium Sulfate. Ind. Eng. Chem. Res. 2011, 50, 9550–9556. [Google Scholar] [CrossRef]
- McAdam, A.C.; Franz, H.B.; Sutter, B.; Archer, P.D.; Freissinet, C.; Eigenbrode, J.L.; Ming, D.W.; Atreya, S.K.; Bish, D.L.; Blake, D.F.; et al. Sulfur-bearing phases detected by evolved gas analysis of the Rocknest aeolian deposit, Gale Crater, Mars. J. Geophys. Res. Planet. 2014, 119, 373–393. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Z.; Liu, H.; Tu, Y.; Yang, W.; Zeng, H.; Qiu, J. Decomposition and solid reactions of calcium sulfate doped with SiO2, Fe2O3 and Al2O3. J. Appl. Anal. Pyrolysis 2015, 113, 491–498. [Google Scholar] [CrossRef]
- ESTA® Kieserit FINE. Available online: https://www.kpluss.com/en-us/our-business-products/agriculture/products/en-esta-kieserit-fein/ (accessed on 15 January 2024).
- Aparecida, C.; Rocha, A.; Cordeiro, G.C.; Toledo Filho, R.D. Use of thermal analysis to determine the hydration products of oil well cement pastes containing NaCl and KCl. J. Therm. Anal. Calorim. 2015, 122, 1279–1288. [Google Scholar] [CrossRef]
- Birks, N. Investigation into the Role of Sodium Chloride Deposited on Oxide and Metal Substrates in the Initiation of Hot Corrosion; University of Pittsburg: Pittsburgh, PA, USA, 1983. [Google Scholar]
- Wiedemann, H.; Smykatz-Kloss, W. Thermal studies of thernardite. Thermochim. Acta 1981, 50, 17–29. [Google Scholar] [CrossRef]
- Rowe, J.J.; Morey, G.W.; Silber, C.C. The ternary system K2SO4·MgSO4·CaSO4. J. Inorg. Nucl. Chem. 1967, 29, 925–942. [Google Scholar] [CrossRef]
- Chou, I.; Sealll, R. Determination of epsomite-hexahydrite equilibria by the humidity-buffer technique at 0.1 MPa with implications for phase equilibria in the system MgSO4-H2O. Astrobiology 2004, 3, 619–630. [Google Scholar] [CrossRef]
- Stein, N.T.; Grotzinger, J.P.; Schieber, J.; Mangold, N.; Hallet, B.; Newsom, H.; Stack, K.M.; Berger, J.A.; Thompson, L.; Sieback, K.L.; et al. Desiccation cracks provide evidence of lake drying on Mars, Sutton Island member, Murray formation, Gale Crater. Geology 2018, 46, 515–518. [Google Scholar] [CrossRef]
- Bonny, S.; Jones, B. Microbes and mineral precipitation, Miette Hot Springs, Jasper National Park, AB, Canada. Can. J. Earth Sci. 2003, 40, 1483–1500. [Google Scholar] [CrossRef]
- Sankaranarayanan, K.; Timofeeff, M.N.; Spathis, R.; Lowenstein, T.K.; Koji Lum, J. Ancient microbes from halite fluid inclusions: Optimized surface sterilization and DNA extraction. PLoS ONE 2011, 6, e20683. [Google Scholar] [CrossRef]
- Douglas, S.; Yang, H. Mineral biosignatures in evaporites: Presence of rosickyite in an endoevaporitic microbial community from Death Valley. Calif. Geol. 2002, 30, 1075–1078. [Google Scholar] [CrossRef]
- Fish, S.A.; Shepherd, T.J.; McGenity, T.J.; Grant, W.D. Recovery of 16S ribosomal RNA gene fragments from ancient halite. Nature 2002, 417, 432–436. [Google Scholar] [CrossRef]
- Kminek, G.; Bada, J.L.; Pogliano, K.; Ward, J.F. Radiation-Dependent Limit for the Viability of Bacterial Spores in Halite Fluid Inclusions and on Mars. Radiat. Res. 2003, 159, 722–729. [Google Scholar] [CrossRef]
- Pavlov, A.; Eigenbrode, J.; Glavin, D.; Floyd, M. Rapid degradation of the organic moleulesin martian surface rocks due to exposure to cosmic rays. Severe implicatiosn to the search of the “extinct” life on Mars. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2023. [Google Scholar]







| Sample | Primary Lithology | Sulfates Detected by CheMin |
|---|---|---|
| Rocknest (RN) | Modern eolian sediment | Anhydrite [16] |
| Gobabeb (GB) | Modern eolian sediment | Anhydrite [16] |
| Ogunquit Beach (OG) | Modern eolian sediment | Anhydrite [18] |
| John Klein (JK) | Lacustrine mudstone | Anhydrite and bassanite [21] |
| Cumberland (CB) | Lacustrine mudstone | Anhydrite and bassanite [21] |
| Windjana (WJ) | Reworked fluvial sandstone | Anhydrite and bassanite [22] |
| Confidence Hills (CH) | Lacustrine mudstone | Jarosite [23] |
| Mojave (MJ) | Lacustrine mudstone | Jarosite [23] |
| Telegraph Peak (TP) | Lacustrine mudstone | Jarosite [23] |
| Buckskin (BK) | Lacustrine mudstone | Anhydrite [23] |
| Oudam (OU) | Lacustrine sand/siltstone | Anhydrite and gypsum [19] |
| Big Sky (BS) | Eolian sandstone | Anhydrite [24] |
| Greenhorn (GH) | Eolian sandstone | Anhydrite and bassanite [24] |
| Marimba (MB) | Lacustrine mudstone | Anhydrite, bassanite, gypsum, and jarosite [19] |
| Quela (QL) | Lacustrine mudstone | Anhydrite, bassanite, gypsum, and jarosite [19] |
| Duluth (DU) | Lacustrine mudstone | Anhydrite and bassanite [17] |
| Stoer (ST) | Lacustrine mudstone | Anhydrite, bassanite, gypsum, and jarosite [17] |
| Highfield (HF) | Lacustrine mudstone | Anhydrite, bassanite, and gypsum [17] |
| Rock Hall (RH) | Lacustrine mudstone | Anhydrite and jarosite [17] |
| Kilmarie (KM) | Lacustrine mudstone | Anhydrite and bassanite [25] |
| Glen Etive (GE) | Lacustrine mudstone | Anhydrite and bassanite [25] |
| Hutton (HU) | Strong diagenetic overprint, grain size indeterminate, and lacustrine | Anhydrite [25] |
| Edinburg (EB) | Eolian sandstone | None [25] |
| Glasgow (GG) | Lacustrine mudstone | Anhydrite and bassanite [25] |
| Mary Anning (MA) | Lacustrine mudstone | Anhydrite and bassanite [25] |
| Groken (GR) | Lacustrine mudstone | Anhydrite and bassanite [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, J.V.; Sutter, B.; McAdam, A.C.; Knudson, C.A.; Casbeer, P.; Tu, V.M.; Rampe, E.B.; Ming, D.W.; Archer, P.D.; Mahaffy, P.R.; et al. Hydrogen Chloride and Sulfur Dioxide Gas Evolutions from the Reaction between Mg Sulfate and NaCl: Implications for the Sample Analysis at the Mars Instrument in Gale Crater, Mars. Minerals 2024, 14, 218. https://doi.org/10.3390/min14030218
Clark JV, Sutter B, McAdam AC, Knudson CA, Casbeer P, Tu VM, Rampe EB, Ming DW, Archer PD, Mahaffy PR, et al. Hydrogen Chloride and Sulfur Dioxide Gas Evolutions from the Reaction between Mg Sulfate and NaCl: Implications for the Sample Analysis at the Mars Instrument in Gale Crater, Mars. Minerals. 2024; 14(3):218. https://doi.org/10.3390/min14030218
Chicago/Turabian StyleClark, Joanna V., Brad Sutter, Amy C. McAdam, Christine A. Knudson, Patrick Casbeer, Valerie M. Tu, Elizabeth B. Rampe, Douglas W. Ming, Paul D. Archer, Paul R. Mahaffy, and et al. 2024. "Hydrogen Chloride and Sulfur Dioxide Gas Evolutions from the Reaction between Mg Sulfate and NaCl: Implications for the Sample Analysis at the Mars Instrument in Gale Crater, Mars" Minerals 14, no. 3: 218. https://doi.org/10.3390/min14030218