Constraints on Ore Genesis from Trace Ore Mineralogy: A New Occurrence of Kupčíkite and Paděraite from the Zhibula Cu Skarn Deposit, Southern Tibet
Abstract
:1. Introduction
2. Geological Setting
3. Sampling and Analytical Methodology
4. Results
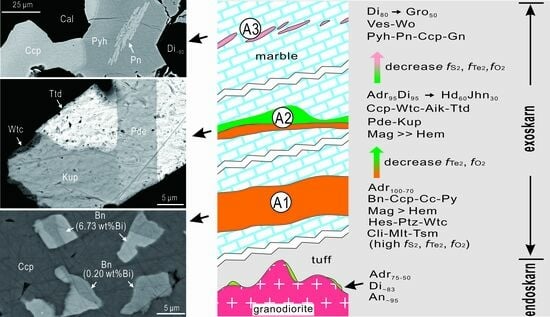
4.1. Ore Petrography and Mineralogy
4.2. Paděraite and Kupčíkite: Micron to Nanoscale Characterization
4.3. Compositional Data
4.3.1. EPMA Data
4.3.2. LA-ICP-MS Trace Element Data
5. Discussion
5.1. A New Skarn Occurrence of Kupčíkite and Paděraite, the First Report from China
5.2. Genetic Constraints—Trace Minerals and Metallogenic Implications
5.2.1. Au-Ag Telluride Associations in Bornite–Chalcopyrite Ores
5.2.2. Bismuth Sulfosalt and Tellurides in Magnetite–Chalcopyrite Ore Prograde to Retrograde Skarn Transition
5.2.3. Pyrrhotite–Chalcopyrite in Distal Skarn
6. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, G.Q.; Li, X.H.; Han, Y.X.; Zhu, Q.Q.; Li, W.; Ye, H.; Song, S.W. Recent progress in study of enrichment mechanism of tellurium, selenium and thallium from oxidized gold-rich porphyry-skarn deposits. Miner. Depos. 2020, 39, 559–567, (In Chinese with English Abstract). [Google Scholar]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulfides, and oxides; Pt. II, applications to telluride-bearing ore deposits. Econ. Geol. 1988, 83, 395–404. [Google Scholar] [CrossRef]
- Cook, N.; Ciobanu, C. Bismuth tellurides and sulphosalts from the Larga hydrothermal system, Metaliferi Mts, Romania: Paragenesis and genetic significance. Mineral. Mag. 2004, 68, 301–321. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Pring, A. Bismuth tellurides as gold scavengers. In Proceedings of the Mineral Deposit Research: Meeting the Global Challenge: Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Mao, J.W., Bierlein, F.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1383–1386. [Google Scholar]
- Topa, D.; Makovicky, E.; Balić-Žunić, T. Crystal structures and crystal chemistry of members of the cuprobismutite homologous series of sulfosalts. Can. Mineral. 2003, 41, 1481–1501. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Pring, A.; Cook, N.J. Micron-to nano-scale intergrowths among members of the cuprobismutite series and paděraite: HRTEM and microanalytical evidence. Mineral. Mag. 2004, 68, 279–300. [Google Scholar] [CrossRef]
- Mumme, W.G.; Žák, L.P. Cu5.9Ag1.3Pb1.6Bi11.2S22, a new mineral of the cuprobismutite-hodrushite group. Neues Jahrb. Mineral. Monatsh. 1985, 12, 557–567. [Google Scholar]
- Topa, D.; Makovicky, E.; Balic-Zunic, T.; Paar, W.H. Kupcikite, Cu3.4Fe0.6Bi5S10, a new Cu-Bi sulfosalt from, Felbertal, Austria, and its crystal structure, Locality: Felbertal, Austria. Can. Mineral. 2003, 41, 1155–1166. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L. Lamellar minerals of the cuprobismutite series and related paděraite: A new occurrence and implications. Can. Mineral. 2003, 41, 441–456. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Topa, D.; Bogdanov, K.; Merkle, R.K.W. Compositional variance in the cuprobismutite series and related (Pb-bearing) paděraite: Insights from new occurrences. In Proceedings of the 18th General Meeting, Edinburgh, UK, 1–6 September 2002; p. 264. [Google Scholar]
- Pršek, J.; Mikus, T.; Makovicky, E.; Chovan, M. Cuprobismutite, kupcíkite, hodrushite and associated sulfosalts from the black shale hosted Ni-Bi-As mineralization at Cierna Lehota, Slovakia. Eur. J. Mineral. 2005, 17, 155–162. [Google Scholar] [CrossRef]
- Pieczka, A.; Gołębiowska, B. Cuprobismutite homologues in granitic pegmatites from Szklarska Poręba, Karkonosze Massif, southwestern Poland. Can. Mineral. 2012, 50, 313–324. [Google Scholar] [CrossRef]
- Izumino, Y.; Nakashima, K.; Nagashima, M. Cuprobismutite group minerals (cuprobismutite, hodrušhite, kupčíkite and padĕraite), other Bi–sulfosalts and Bi–tellurides from the Obari mine, Yamagata Prefecture, Japan. J. Mineral. Petrol. Sci. 2014, 109, 177–190. [Google Scholar] [CrossRef]
- Makovicky, E. Modular classification of sulphosalts: Current status, definition and application of homologous series. Neues Jahrb. Mineral. Abh. 1989, 160, 269–297. [Google Scholar]
- Hou, Z.Q.; Duan, L.F.; Lu, Y.J.; Zheng, Y.C.; Zhu, D.C.; Yang, Z.M.; Yang, Z.S.; Wang, B.D.; Pei, Y.R.; Zhao, Z.D. Lithospheric architecture of the Lhasa Terrane and its control on ore deposits in the Himalayan-Tibetan Orogen. Econ. Geol. 2015, 110, 1541–1575. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Yang, Z.M.; Lu, Y.J.; Kemp, A.; Zheng, Y.C.; Li, Q.Y.; Tang, J.X.; Yang, Z.S.; Duan, L.F. A genetic linkage between subduction-and collision-related porphyry Cu deposits in continental collision zones. Geology 2015, 43, 247–250. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.J.; Li, G.M.; Evans, N.J.; Silang, W.D.; Qin, K.Z. Tracing the genesis of the Zhibula skarn deposit, Tibet, using Co, Te, Au and Ag occurrence. Ore Geol. Rev. 2023, 160, 105601. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.J.; Li, G.M.; Shan, P.F.; Qin, K.Z. The occurrence of cobalt and implications for genesis of the Pusangguo cobalt-rich skarn deposit in Gangdese, Tibet. Ore Geol. Rev. 2022, 150, 105193. [Google Scholar] [CrossRef]
- Dong, Y.H.; Xu, J.F.; Zeng, Q.G.; Mao, G.Z.; Li, J. Is there a Neo-Tethys’ subduction record earlier than arc volcanic rocks in the Sangri group? Acta Petrol. Sin. 2006, 22, 661–668, (In Chinese with English Abstract). [Google Scholar]
- Hu, Y.B.; Liu, J.Q.; Ling, M.X.; Ding, W.; Liu, Y.; Zartman, R.E.; Sun, W.D. The formation of Qulong adakites and their relationship with porphyry copper deposit: Geochemical constraints. Lithos 2015, 220, 60–80. [Google Scholar] [CrossRef]
- Li, G.M.; Rui, Z.Y.; Wang, G.M.; Lin, F.C.; Liu, B.; She, H.Q.; Feng, C.Y.; Qu, W.J. Molybdenite Re-Os dating of Jiama and Zhibula polymetallic copper deposits in Gangdese metallogenic belt of Tibet and its significance. Mineral. Depos. 2005, 24, 481–489, (In Chinese with English Abstract). [Google Scholar]
- Xu, J.; Zheng, Y.Y.; Sun, X.; Shen, Y.H. Alteration and mineralization at the Zhibula Cu skarn deposit, Gangdese belt, Tibet. Ore Geol. Rev. 2016, 75, 304–326. [Google Scholar] [CrossRef]
- Xu, J.; Ciobanu, C.L.; Cook, N.J.; Zheng, Y.Y.; Sun, X.; Wade, B.P. Skarn formation and trace elements in garnet and associated minerals from Zhibula copper deposit, Gangdese Belt, southern Tibet. Lithos 2016, 262, 213–231. [Google Scholar] [CrossRef]
- Xu, J.; Ciobanu, C.L.; Cook, N.J.; Slattery, A. Crystals from the powellite-scheelite series at the nanoscale: A case study from the Zhibula Cu skarn, Gangdese Belt, Tibet. Minerals 2019, 9, 340. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E. The crystal structure of paderaite, Cu7 (X 0.33 Pb1.33Bi11.33)∑13S22, with X = Cu or Ag: New data and interpretation. Can. Mineral. 2006, 44, 481–495. [Google Scholar] [CrossRef]
- Becker, M.; Bradshaw, D.; De Villiers, J. The mineralogy of pyrrhotite from Sudbury CCN and Phoenix nickel ores and its effect on flotation performance. Can. Metal. Quart. 2011, 50, 10–19. [Google Scholar] [CrossRef]
- Mumme, W.G. The crystal structure of padĕraite, a mineral of the cuprobismutite series. Can. Mineral. 1986, 24, 513–521. [Google Scholar]
- Mariolacos, K. Phase relations in the system Bi2S3-PbS-CuPbBiS3 at 450 °C and its extension in the system Bi2S3-PbS-Cu2S. Neues. Jahrb. Mineral. Monatsh. 1979, 13, 73–80. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J. Skarn textures: A case study of the Ocna de Fier-Dognecea orefield, Banat, Romania. Ore Geol. Rev. 2004, 24, 315–370. [Google Scholar] [CrossRef]
- Meinert, L.D. Gold in skarns related to epizonal intrusions. Rev. Econ. Geol. 2000, 13, 347–375. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L. Paragenesis of Cu-Fe ores from Ocna de Fier-Dognecea (Romania), typifying fluid plume mineralization in a proximal skarn setting. Mineral. Mag. 2001, 65, 351–372. [Google Scholar] [CrossRef]
- Bowman, J.R. Basic aspects and applications of phase equilibria in the analysis of metasomatic Ca–Mg–Al–Fe–Si skarns. In Mineralized Intrusion Related Skarn Systems; Lentz, D.R., Ed.; Mineralogical Association of Canada Short Course; Mineralogical Association of Canada: Ottawa, ON, Canada, 1998; Volume 26, pp. 1–49. [Google Scholar]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. Repr. Ser. 1992, 6, 117–134. [Google Scholar]
- Dipple, G.M.; Gerdes, M.L. Reaction-infiltration feedback and hydrodynamics at the skarn front. In Mineralized Intrusion-Related Skarn Systems; Lentz, D.R., Ed.; Mineralogical Association of Canada Short Course; Mineralogical Association of Canada: Ottawa, ON, Canada, 1998; Volume 26, pp. 71–97. [Google Scholar]
- Mueller, A.G.; Hagemann, S.G.; Jol, B.; Yanlu, X.; Roberts, M.P. Early Fimiston and late Oroya Au–Te ore, Paringa south mine, golden mile, Kalgoorlie: 4. Mineralogical and thermodynamic constraints on gold deposition by magmatic fluids at 420–300 °C and 300 Mpa. Mineral. Depos. 2020, 55, 767–796. [Google Scholar] [CrossRef]
- Oen, I.S.; Kieft, C. Silver-bearing wittichenite-chalcopyrite-bornite intergrowths and associated minerals in the Mangualade pegmatite, Portugal. Can. Mineral. 1976, 14, 185–193. [Google Scholar]
- Shalaby, I.M.; Stumpfl, E.; Helmy, H.M.; El Mahallawi, M.M.; Kamel, O.A. Silver and silver-bearing minerals at the Um Samiuki volcanogenic massive sulphide deposit, Eastern Desert, Egypt. Mineral. Depos. 2004, 39, 608–621. [Google Scholar] [CrossRef]
- Novoselov, K.A.; Belogub, E.V.; Zaykov, V.V.; Yakovlev, V.A. Silver sulfosalts from volcanic-hosted massive sulphide deposits in the Southern Urals. Mineral. Petrol. 2006, 87, 327–349. [Google Scholar] [CrossRef]
- Jian, W.; Lehmann, B.; Mao, J.; Ye, H.; Li, Z.; Zhang, J.; Ye, Y. Telluride and Bi-sulfosalt mineralogy of the Yangzhaiyu gold deposit, Xiaoqinling region, Central China. Can. Mineral. 2014, 52, 883–898. [Google Scholar] [CrossRef]
- Sugaki, A.; Kitakaze, A.; Harada, H. Hydrothermal synthesis and phase relations of the polymetallic sulfide system, especially on the Cu-Fe-Bi-S system. In Materials Science of the Earth’s Interior; Sunagawa, I., Ed.; Terra Publication: Tokyo, Japan, 1984; pp. 545–583. [Google Scholar]
- Nanri, H.; Sugaki, A.; Shima, H.; Kitakaze, A. Phase relations of Cu-Fe-Bi-S system (V) bornite solid solution field at 400 °C and 500 °C (abstr.). J. Jpn. Assoc. Miner. Petrol. Econ. Geol. 1978, 73, 83. (In Japanese) [Google Scholar]
- Dobbe, R.T.M.; Oen, I.S. The polymetallic Cu-Co ores in the central mineralised zone at Tunaberg, Bergslagen, Sweden. Neues Jahrb. Mineral. Abh. 1994, 166, 261–294. [Google Scholar]











| Skarn Type | Sample ID | Drill Hole | Depth (m) | Main Skarn Assemblage | Minor Skarn Assemblage | Ore Minerals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grt | Px | Czo | Act | Chl | Qz | Cal | Sulfides | Oxides | |||||
| Distal skarn (banded Grt-Px in marble) | 321 | 1611 | 321 | xx | x | x | x | xxx | Wo, Ap, Ttn, Ves | Pyh, Ccp, Gn, Sp, Pn | |||
| 323 | 1611 | 323 | xx | x | x | xxx | Wo, Ap, An, Ves | Pyh, Ccp, Gn, Sp, Pn | |||||
| Proximal skarn (massive Grt) | 372 | 1611 | 372 | xxx | x | x | x | x | x | Ap | Ccp, Bn, Mol, Cli, Gn, Sp, Py | Mag, Hem, Sch | |
| 374 | 1611 | 374 | xxx | x | x | x | x | Ap | Ccp, Bn, Cc, Sp, Gn, Ptz, Hes, Wtc, Cli | ||||
| 8 | quarry | - | xxx | x | x | x | x | Ap | Bn, Ccp, Mol, Cc, Gn, Hes, Ptz, El, Cli | Sch | |||
| Proximal skarn (retrogressed Px in contact with marble) | 180 | 1611 | 180 | x | xxxx | x | x | xx | xxx | Ap, Wo | Ccp, Bn, Wtc, Aik, Ttd, Pde, Kup | Mag, Hem, Sch | |
| 355 | 1215 | 355 | x | xx | xxx | xx | xx | xxx | xx | Ap | Ccp, Sp, Gn | ||
| Bi-Rich Bornite-like Phase (wt.%) | Normal Bornite (wt.%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 374 (n = 3) | Sample 374 (n = 13) | Sample 8 (n = 29) | Sample 180 (n = 3) | Sample 372 (n = 2) | ||||||||||||||||
| 1 | 2 | 3 | Mean | Max. | Min. | SD | Mean | Max. | Min. | SD | Mean | Max. | Min. | SD | Mean | Max. | Min. | SD | Mean | |
| Cu | 54.6 | 55.24 | 54.77 | 54.87 | 64.50 | 61.39 | 0.73 | 63.55 | 63.90 | 62.50 | 0.37 | 63.35 | 60.86 | 60.59 | 0.11 | 60.71 | 62.93 | 62.52 | 0.20 | 62.72 |
| Ag | 0.09 | 0.09 | 0.07 | 0.08 | 0.20 | <dl | 0.06 | 0.03 | 0.23 | <dl | 0.04 | 0.12 | 0.06 | <dl | 0.00 | 0.02 | 0.10 | 0.09 | 0.00 | 0.10 |
| Fe | 14.53 | 14.12 | 13.68 | 14.11 | 11.59 | 10.27 | 0.31 | 11.31 | 11.48 | 10.78 | 0.17 | 11.19 | 15.23 | 14.76 | 0.20 | 14.96 | 11.65 | 11.64 | 0.00 | 11.64 |
| Pb | <dl | 0.07 | <dl | 0.02 | 0.10 | <dl | 0.02 | 0.04 | 0.25 | <dl | 0.05 | 0.05 | <dl | <dl | - | <dl | 0.09 | <dl | 0.03 | 0.05 |
| Bi | 6.47 | 6.82 | 6.9 | 6.73 | 0.51 | 0.12 | 0.10 | 0.20 | 0.31 | 0.11 | 0.04 | 0.18 | 0.30 | 0.25 | 0.02 | 0.27 | 0.39 | 0.25 | 0.07 | 0.32 |
| Co | 26.6 | 26.5 | 26.32 | 0.01 | 0.03 | <dl | 0.01 | 0.01 | 0.04 | <dl | 0.01 | 0.01 | 0.17 | 0.08 | 0.04 | 0.14 | 0.02 | <dl | 0.01 | 0.01 |
| S | 0.07 | 0.07 | 0.09 | 26.47 | 25.64 | 23.38 | 0.51 | 25.02 | 27.74 | 25.77 | 0.35 | 26.35 | 26.83 | 26.22 | 0.27 | 26.60 | 26.23 | 26.13 | 0.05 | 26.18 |
| Se | <dl | 0.02 | 0.02 | 0.08 | 0.09 | <dl | 0.01 | 0.04 | 0.09 | <dl | 0.02 | 0.03 | 0.05 | <dl | 0.00 | 0.02 | 0.06 | <dl | 0.02 | 0.03 |
| Total | 102.35 | 102.91 | 101.83 | 102.36 | 101.14 | 98.59 | 0.74 | 100.22 | 102.08 | 99.91 | 0.62 | 101.27 | 102.61 | 102.52 | 0.04 | 102.58 | 101.34 | 100.77 | 0.28 | 101.06 |
| Formula (to 10 atoms) | ||||||||||||||||||||
| Cu | 4.336 | 4.383 | 4.391 | 4.370 | 5.167 | 4.938 | 0.058 | 5.038 | 4.978 | 4.820 | 0.031 | 4.931 | 4.672 | 4.632 | 0.017 | 4.650 | 4.901 | 4.894 | 0.004 | 4.898 |
| Ag | 0.004 | 0.004 | 0.004 | 0.004 | 0.009 | - | 0.003 | 0.002 | 0.011 | - | 0.002 | 0.005 | 0.003 | - | 0.000 | 0.001 | 0.005 | 0.004 | 0.000 | 0.004 |
| Pb | - | 0.002 | - | 0.001 | 0.002 | - | 0.001 | 0.001 | 0.006 | - | 0.001 | 0.001 | - | - | - | - | 0.002 | - | 0.000 | 0.001 |
| Fe | 1.313 | 1.275 | 1.248 | 1.279 | 1.058 | 0.926 | 0.029 | 1.020 | 1.009 | 0.950 | 0.012 | 0.991 | 1.331 | 1.286 | 0.020 | 1.303 | 1.037 | 1.032 | 0.002 | 1.035 |
| Bi | 0.156 | 0.165 | 0.168 | 0.163 | 0.012 | 0.003 | 0.002 | 0.005 | 0.007 | 0.003 | 0.001 | 0.004 | 0.007 | 0.006 | 0.000 | 0.006 | 0.009 | 0.006 | 0.002 | 0.008 |
| Total M | 5.809 | 5.828 | 5.811 | 5.816 | 6.232 | 5.998 | 0.055 | 6.065 | 5.991 | 5.779 | 0.037 | 5.933 | 6.010 | 5.934 | 0.035 | 5.960 | 5.946 | 5.945 | 0.001 | 5.945 |
| S | 4.186 | 4.167 | 4.183 | 4.179 | 3.997 | 3.768 | 0.054 | 3.932 | 4.221 | 4.006 | 0.038 | 4.065 | 4.066 | 3.990 | 0.034 | 4.039 | 4.055 | 4.050 | 0.003 | 4.053 |
| Se | 0.004 | 0.005 | 0.006 | 0.005 | 0.006 | - | 0.001 | 0.003 | 0.006 | - | 0.001 | 0.002 | 0.003 | - | 0.000 | 0.001 | 0.004 | - | 0.000 | 0.002 |
| S(+Se) | 4.191 | 4.172 | 4.189 | 4.184 | 4.002 | 3.768 | 0.055 | 3.935 | 4.221 | 4.009 | 0.037 | 4.067 | 4.066 | 3.990 | 0.035 | 4.040 | 4.055 | 4.054 | 0.001 | 4.055 |
| M/S | 1.386 | 1.397 | 1.387 | 1.390 | 1.654 | 1.499 | 0.037 | 1.542 | 1.495 | 1.369 | 0.022 | 1.459 | 1.506 | 1.460 | 0.022 | 1.476 | 1.467 | 1.466 | 0.001 | 1.466 |
| Wittichenite (Sample 374, n = 4) | Wittichenite (Sample 180, n = 10) | Aikinite (Sample 180, n = 11) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. | Min. | SD | Mean | Max. | Min. | SD | Mean | Max. | Min. | SD | Mean | |
| Cu | 39.80 | 38.79 | 0.38 | 39.35 | 38.03 | 36.88 | 0.39 | 38.00 | 10.98 | 10.54 | 0.13 | 10.75 |
| Ag | 0.50 | 0.30 | 0.07 | 0.39 | 0.22 | <dl | 0.05 | 0.19 | <dl | <dl | - | <dl |
| Fe | 1.16 | 0.60 | 0.22 | 0.83 | 0.87 | 0.54 | 0.14 | 0.77 | 0.86 | 0.07 | 0.29 | 0.53 |
| Cd | 0.09 | 0.06 | 0.01 | 0.08 | 0.06 | 0.04 | 0.01 | 0.04 | 0.14 | 0.05 | 0.04 | 0.04 |
| Pb | <dl | <dl | - | <dl | <dl | <dl | - | <dl | 36.22 | 35.11 | 0.37 | 35.53 |
| Mn | <dl | <dl | - | <dl | 0.08 | <dl | 0.02 | 0.02 | 0.03 | <dl | 0.01 | 0.01 |
| Bi | 40.42 | 39.15 | 0.51 | 39.55 | 41.89 | 40.57 | 0.44 | 40.90 | 37.56 | 36.07 | 0.46 | 37.01 |
| S | 20.12 | 19.80 | 0.12 | 19.94 | 20.45 | 19.39 | 0.30 | 20.06 | 16.91 | 15.70 | 0.39 | 16.25 |
| Te | <dl | <dl | - | <dl | <dl | <dl | - | <dl | 0.02 | <dl | 0.01 | 0.01 |
| Se | 0.11 | <dl | 0.02 | 0.07 | 1.04 | 0.09 | 0.25 | 0.26 | 1.07 | 0.32 | 0.29 | 0.67 |
| Total | 101.44 | 98.97 | 0.98 | 100.18 | 101.45 | 98.41 | 0.87 | 99.87 | 102.11 | 99.59 | 0.91 | 100.64 |
| Formula (to 7 atoms) | Formula (to 6 atoms) | |||||||||||
| Cu | 3.004 | 2.977 | 0.011 | 2.989 | 2.922 | 2.870 | 0.016 | 2.923 | 1.157 | 0.947 | 0.078 | 1.050 |
| Ag | 0.022 | 0.013 | 0.003 | 0.017 | 0.010 | - | 0.002 | 0.009 | - | - | - | - |
| Pb | - | - | - | - | - | - | - | - | 1.185 | 0.978 | 0.080 | 1.064 |
| Fe | 0.100 | 0.053 | 0.018 | 0.072 | 0.075 | 0.047 | 0.012 | 0.033 | 0.101 | 0.008 | 0.033 | 0.041 |
| Cd | 0.004 | 0.003 | 0.001 | 0.003 | 0.003 | 0.002 | 0.000 | 0.002 | 0.008 | 0.003 | 0.002 | 0.002 |
| Cu+Ag+Fe | 3.126 | 3.047 | 0.029 | 3.078 | 2.986 | 2.875 | 0.031 | 2.965 | 1.202 | 1.007 | 0.075 | 1.091 |
| Pb+Cd | 0.004 | 0.003 | 0.001 | 0.003 | 0.003 | 0.002 | 0.000 | 0.002 | 1.188 | 0.984 | 0.081 | 1.066 |
| Bi | 0.925 | 0.904 | 0.008 | 0.914 | 0.989 | 0.959 | 0.011 | 0.957 | 1.204 | 1.010 | 0.084 | 1.099 |
| Bi+Sb+As | 0.925 | 0.904 | 0.008 | 0.914 | 0.989 | 0.959 | 0.011 | 0.957 | 1.204 | 1.010 | 0.084 | 1.099 |
| Total M | 4.034 | 3.968 | 0.026 | 3.994 | 3.947 | 3.867 | 0.023 | 3.924 | 3.549 | 3.022 | 0.235 | 3.256 |
| S | 3.028 | 2.963 | 0.025 | 3.002 | 3.120 | 2.988 | 0.035 | 3.060 | 3.470 | 2.871 | 0.264 | 3.145 |
| Te | - | - | - | - | - | - | - | - | 0.001 | - | 0.000 | 0.001 |
| Se | 0.007 | - | 0.001 | 0.005 | 0.065 | 0.006 | 0.015 | 0.016 | 0.092 | 0.027 | 0.024 | 0.053 |
| S(+Te+Se) | 3.032 | 2.966 | 0.026 | 3.006 | 3.133 | 3.053 | 0.023 | 3.076 | 3.507 | 2.943 | 0.264 | 3.198 |
| Charge M | 5.946 | 5.850 | 0.042 | 5.896 | 5.922 | 5.808 | 0.037 | 5.874 | 7.145 | 6.071 | 0.478 | 6.562 |
| Charge S | 6.063 | 5.932 | 0.051 | 6.012 | 6.266 | 6.106 | 0.046 | 6.152 | 7.014 | 5.885 | 0.528 | 6.396 |
| mean | 5.965 | 5.939 | 0.011 | 5.954 | 6.057 | 6.014 | 0.013 | 6.013 | 7.037 | 6.005 | 0.502 | 6.479 |
| diff. | 0.014 | −0.204 | 0.091 | −0.116 | −0.185 | −0.437 | 0.079 | −0.278 | 0.292 | 0.004 | 0.087 | 0.166 |
| diff (%) | 0.2 | −3.4 | 1.5 | −1.9 | −3.1 | −7.2 | 1.3 | −4.6 | 4.8 | 0.1 | 1.5 | 2.6 |
| Pb | Bi | Cu | Ag | Fe | Cd | S | Se | Te | Total | Pb | Bi | Ag | Cu | Fe | Cd | S | Se | Te | eV% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kupčíkite | Formula (to 19 atoms) | |||||||||||||||||||
| Ku-1 | 0.46 | 64.90 | 13.33 | 0.21 | 2.33 | 0.23 | 19.81 | 0.63 | <dl | 101.9 | 0.035 | 4.941 | 0.031 | 3.337 | 0.664 | 0.033 | 9.832 | 0.127 | - | −1.3 |
| Ku-2 | 0.52 | 64.37 | 13.09 | 0.19 | 2.14 | 0.29 | 19.72 | 0.65 | <dl | 100.97 | 0.040 | 4.949 | 0.028 | 3.310 | 0.616 | 0.041 | 9.883 | 0.132 | - | −2.3 |
| Ku-3 | 0.26 | 64.17 | 13.39 | 0.11 | 2.00 | 0.27 | 19.57 | 0.68 | <dl | 100.45 | 0.020 | 4.956 | 0.016 | 3.400 | 0.578 | 0.039 | 9.851 | 0.139 | - | −2.1 |
| Ku-4 | <dl | 64.22 | 13.08 | 0.13 | 2.19 | 0.34 | 19.66 | 0.70 | <dl | 100.32 | - | 4.954 | 0.019 | 3.318 | 0.632 | 0.049 | 9.885 | 0.143 | - | −2.5 |
| Ku-5 | 0.21 | 64.21 | 13.73 | 0.19 | 2.17 | 0.26 | 19.61 | 0.64 | <dl | 101.02 | 0.016 | 4.918 | 0.028 | 3.458 | 0.622 | 0.037 | 9.791 | 0.130 | - | −1.3 |
| Ku-6 | <dl | 63.82 | 13.52 | <dl | 2.32 | 0.20 | 19.45 | 0.71 | <dl | 100.02 | - | 4.929 | - | 3.434 | 0.671 | 0.029 | 9.792 | 0.145 | - | −1.3 |
| Ku-7 | 0.39 | 63.95 | 13.68 | 0.15 | 2.26 | 0.22 | 19.77 | 0.63 | <dl | 101.05 | 0.030 | 4.879 | 0.022 | 3.432 | 0.645 | 0.031 | 9.832 | 0.127 | - | −2.1 |
| Ku-8 | 0.04 | 64.06 | 13.54 | 0.14 | 2.21 | 0.22 | 19.67 | 0.60 | <dl | 100.48 | 0.003 | 4.920 | 0.021 | 3.420 | 0.635 | 0.031 | 9.848 | 0.122 | - | −2.0 |
| Ku-9 | <dl | 64.39 | 13.35 | 0.09 | 2.34 | 0.26 | 19.46 | 0.68 | <dl | 100.57 | - | 4.966 | 0.013 | 3.386 | 0.675 | 0.037 | 9.783 | 0.139 | - | −0.6 |
| Ku-10 | <dl | 64.21 | 13.70 | 0.08 | 2.53 | 0.10 | 19.71 | 0.70 | <dl | 101.03 | - | 4.892 | 0.012 | 3.432 | 0.721 | 0.014 | 9.788 | 0.141 | - | −1.4 |
| Ku-11 | 0.23 | 64.64 | 13.23 | <dl | 2.39 | 0.12 | 19.57 | 0.75 | <dl | 100.93 | 0.018 | 4.970 | - | 3.345 | 0.688 | 0.017 | 9.809 | 0.153 | - | −1.1 |
| Ku-12 | 0.51 | 63.34 | 13.27 | <dl | 1.94 | 0.06 | 19.34 | 0.63 | <dl | 99.09 | 0.040 | 4.961 | - | 3.418 | 0.569 | 0.009 | 9.873 | 0.131 | - | −2.4 |
| 180Ku-13 | 0.77 | 63.99 | 13.45 | <dl | 1.76 | 0.11 | 18.98 | 0.79 | <dl | 99.85 | 0.061 | 5.032 | - | 3.478 | 0.518 | 0.016 | 9.730 | 0.164 | - | −0.1 |
| Ku-14 | 0.68 | 63.30 | 13.69 | <dl | 1.51 | 0.14 | 18.90 | 0.77 | <dl | 98.99 | 0.054 | 5.008 | - | 3.562 | 0.447 | 0.021 | 9.747 | 0.161 | - | −0.9 |
| Ku-15 | 0.06 | 63.09 | 13.41 | 0.13 | 1.34 | 0.08 | 18.85 | 0.72 | <dl | 97.68 | 0.005 | 5.048 | 0.020 | 3.529 | 0.401 | 0.012 | 9.832 | 0.152 | - | −2.2 |
| Ku-16 | 0.85 | 63.77 | 13.79 | <dl | 1.51 | 0.17 | 19.32 | 0.79 | <dl | 100.2 | 0.067 | 4.966 | - | 3.532 | 0.440 | 0.025 | 9.808 | 0.163 | - | −2.3 |
| Ku-17 | 0.08 | 63.29 | 13.07 | 0.10 | 1.93 | 0.11 | 18.92 | 0.70 | <dl | 98.2 | 0.006 | 5.028 | 0.015 | 3.415 | 0.574 | 0.016 | 9.798 | 0.147 | - | −0.9 |
| Ku-18 | 0.14 | 64.28 | 13.99 | <dl | 1.97 | 0.09 | 19.67 | 0.72 | <dl | 100.86 | 0.011 | 4.923 | - | 3.523 | 0.565 | 0.013 | 9.820 | 0.146 | - | −2.3 |
| Min. | <dl | 63.09 | 13.07 | <dl | 1.34 | 0.06 | 18.85 | 0.60 | <dl | 97.68 | 4.879 | 3.310 | 0.401 | 0.009 | 9.730 | 0.122 | - | −2.5 | ||
| Max. | 0.85 | 64.90 | 13.99 | 0.21 | 2.53 | 0.34 | 19.81 | 0.79 | <dl | 101.90 | 0.067 | 5.048 | 0.031 | 3.562 | 0.721 | 0.049 | 9.885 | 0.164 | - | −0.1 |
| Mean | 0.29 | 64.00 | 13.46 | 0.08 | 2.05 | 0.18 | 19.44 | 0.69 | 100.20 | 0.02 | 4.96 | 0.01 | 3.43 | 0.59 | 0.03 | 9.82 | 0.14 | −1.6 | ||
| SD | 0.26 | 0.48 | 0.26 | 0.04 | 0.32 | 0.08 | 0.31 | 0.06 | 1.05 | 0.02 | 0.05 | 0.01 | 0.07 | 0.09 | 0.01 | 0.04 | 0.01 | 0.7 | ||
| Paděraite | Formula (to 42 atoms) | |||||||||||||||||||
| Pad-1 | 7.25 | 62.26 | 11.48 | 0.36 | 0.51 | 0.12 | 18.64 | 0.98 | 0.13 | 101.73 | 1.310 | 11.153 | 0.125 | 6.762 | 0.342 | 0.040 | 21.765 | 0.465 | 0.038 | −1.8 |
| Pad-2 | 7.29 | 61.92 | 11.58 | 0.28 | 0.39 | 0.05 | 18.57 | 0.96 | 0.18 | 101.22 | 1.324 | 11.146 | 0.098 | 6.855 | 0.263 | 0.017 | 21.789 | 0.457 | 0.053 | −2.3 |
| Pad-3 | 7.58 | 61.70 | 11.66 | 0.30 | 0.38 | 0.09 | 18.31 | 1.02 | 0.19 | 101.23 | 1.383 | 11.159 | 0.105 | 6.935 | 0.257 | 0.030 | 21.586 | 0.488 | 0.056 | −0.9 |
| Pad-4 | 7.35 | 61.07 | 11.35 | 0.27 | 0.68 | 0.09 | 18.32 | 1.09 | 0.15 | 100.37 | 1.344 | 11.075 | 0.095 | 6.769 | 0.461 | 0.030 | 21.657 | 0.523 | 0.045 | −1.6 |
| Pad-5 | 7.25 | 61.87 | 11.36 | 0.25 | 0.47 | 0.09 | 18.08 | 1.01 | 0.16 | 100.54 | 1.337 | 11.311 | 0.089 | 6.829 | 0.322 | 0.031 | 21.546 | 0.489 | 0.048 | 0.1 |
| Pad-6 | 7.58 | 61.76 | 11.16 | 0.24 | 0.47 | 0.12 | 18.48 | 0.98 | 0.22 | 101.01 | 1.384 | 11.182 | 0.084 | 6.645 | 0.318 | 0.040 | 21.811 | 0.470 | 0.065 | −2.1 |
| Pad-7 | 7.12 | 61.70 | 11.20 | 0.25 | 1.09 | 0.07 | 18.06 | 0.99 | 0.08 | 100.56 | 1.306 | 11.224 | 0.088 | 6.700 | 0.742 | 0.024 | 21.415 | 0.477 | 0.024 | 1.7 |
| Pad-8 | 6.92 | 61.91 | 12.87 | 0.21 | 0.50 | <dl | 18.14 | 0.92 | 0.18 | 101.65 | 1.250 | 11.090 | 0.073 | 7.581 | 0.335 | - | 21.181 | 0.436 | 0.053 | 1.7 |
| Pad-9 | 6.85 | 61.93 | 12.47 | 0.25 | 0.27 | <dl | 18.45 | 0.97 | 0.16 | 101.35 | 1.238 | 11.095 | 0.087 | 7.347 | 0.181 | - | 21.546 | 0.460 | 0.047 | −1.3 |
| Pad-10 | 7.37 | 61.15 | 12.80 | 0.11 | 0.39 | 0.06 | 18.64 | 0.97 | 0.16 | 101.65 | 1.319 | 10.846 | 0.038 | 7.466 | 0.259 | 0.020 | 21.551 | 0.455 | 0.046 | −2.0 |
| Pad-11 | 7.26 | 61.15 | 12.44 | 0.28 | 0.45 | 0.05 | 18.45 | 0.96 | 0.16 | 101.20 | 1.310 | 10.940 | 0.097 | 7.318 | 0.301 | 0.017 | 21.515 | 0.455 | 0.047 | −1.2 |
| Pad-12 | 6.93 | 61.22 | 12.56 | 0.29 | 0.42 | 0.11 | 18.18 | 1.02 | 0.19 | 100.92 | 1.258 | 11.018 | 0.101 | 7.433 | 0.283 | 0.037 | 21.328 | 0.486 | 0.056 | 0.0 |
| Min. | 6.85 | 61.07 | 11.16 | 0.11 | 0.27 | <dl | 18.06 | 0.92 | 0.08 | 100.37 | 1.238 | 10.846 | 0.038 | 6.645 | 0.181 | - | 21.181 | 0.436 | 0.024 | −2.3 |
| Max. | 7.58 | 62.26 | 12.87 | 0.36 | 1.09 | 0.12 | 18.64 | 1.09 | 0.22 | 101.73 | 1.384 | 11.311 | 0.125 | 7.581 | 0.742 | 0.040 | 21.811 | 0.523 | 0.065 | 1.7 |
| Mean | 7.23 | 61.64 | 11.91 | 0.26 | 0.50 | 0.07 | 18.36 | 0.99 | 0.16 | 101.12 | 1.314 | 11.103 | 0.090 | 7.053 | 0.339 | 0.024 | 21.558 | 0.472 | 0.048 | −0.8 |
| SD | 0.23 | 0.37 | 0.63 | 0.06 | 0.20 | 0.03 | 0.20 | 0.04 | 0.03 | 0.44 | 0.045 | 0.120 | 0.020 | 0.330 | 0.137 | 0.009 | 0.180 | 0.022 | 0.010 | 1.3 |
| Cu | Ag | Cd | Pb | Bi | S | Te | Se | Total | Cu | Ag | Pb | Cd | Cu+Ag | Pb+Cd | Bi | Total M | S | Te | Se | S(+Te+Se) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tetradymite (sample 180, n = 15) | Formula (to 5 atoms) | ||||||||||||||||||||
| Max. | 0.13 | 0.22 | 0.14 | 0.13 | 59.51 | 5.05 | 37.21 | 2.17 | 101.65 | 0.015 | 0.014 | 0.004 | 0.009 | 0.022 | 0.009 | 1.971 | 1.983 | 1.090 | 2.012 | 0.191 | 3.077 |
| Min. | <dl | <dl | <dl | <dl | 56.83 | 4.16 | 34.33 | 0.74 | 98.51 | - | - | - | - | - | - | 1.910 | 1.923 | 0.917 | 1.862 | 0.065 | 3.017 |
| SD | 0.02 | 0.04 | 0.03 | 0.03 | 0.73 | 0.28 | 0.80 | 0.40 | 0.99 | 0.003 | 0.003 | 0.001 | 0.002 | 0.005 | 0.002 | 0.014 | 0.014 | 0.052 | 0.042 | 0.036 | 0.014 |
| Mean | 0.08 | 0.06 | 0.04 | 0.05 | 58.39 | 4.69 | 35.48 | 1.44 | 100.24 | 0.009 | 0.004 | 0.002 | 0.003 | 0.012 | 0.004 | 1.928 | 1.945 | 1.010 | 1.919 | 0.126 | 3.055 |
| S | Se | Cu | Ag | Hg | Te | Bi | Au | Total | S | Se | Te | S+Se+Te | Cu | Ag | Hg | Bi | Au | Total M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hessite (sample 8, n = 7) | Formula (to 3 atoms) | ||||||||||||||||||
| Max. | 0.11 | 0.19 | 0.53 | 63.82 | 0.22 | 40.83 | 0.07 | 0.32 | 102.53 | 0.011 | 0.008 | 1.097 | 1.111 | 0.028 | 1.988 | 0.004 | 0.001 | 0.006 | 2.005 |
| Min. | 0.04 | 0.00 | <dl | 59.35 | <dl | 37.37 | <dl | <dl | 100.53 | 0.005 | 0.002 | 0.984 | 0.995 | - | 1.886 | - | - | - | 1.886 |
| SD | 0.02 | 0.05 | 0.11 | 1.49 | 0.04 | 1.12 | 0.01 | 0.09 | 0.69 | 0.002 | 0.002 | 0.037 | 0.038 | 0.009 | 0.032 | 0.001 | 0.000 | 0.001 | 0.038 |
| Mean | 0.07 | 0.08 | 0.36 | 62.93 | 0.10 | 38.20 | 0.02 | 0.07 | 101.73 | 0.007 | 0.003 | 1.008 | 1.019 | 0.014 | 1.964 | 0.002 | - | 0.001 | 1.981 |
| Hessite (sample 374, n = 4) | Formula (to 3 atoms) | ||||||||||||||||||
| Max. | 0.20 | 0.26 | <dl | 64.14 | 0.22 | 37.91 | 0.07 | 0.34 | 102.82 | 0.021 | 0.011 | 0.991 | 1.016 | - | 2.000 | 0.004 | 0.001 | 0.006 | 2.007 |
| Min. | 0.12 | 0.12 | <dl | 63.36 | 0.12 | 36.23 | <dl | <dl | 100.38 | 0.012 | 0.005 | 0.967 | 0.993 | - | 1.978 | 0.002 | - | - | 1.984 |
| SD | 0.03 | 0.06 | - | 0.28 | 0.04 | 0.69 | 0.00 | 0.07 | 0.93 | 0.003 | 0.003 | 0.010 | 0.010 | - | 0.011 | 0.001 | 0.000 | 0.002 | 0.010 |
| Mean | 0.16 | 0.19 | <dl | 63.75 | 0.15 | 37.19 | 0.02 | 0.18 | 101.64 | 0.017 | 0.008 | 0.981 | 1.005 | - | 1.989 | 0.003 | 0.000 | 0.003 | 1.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Ciobanu, C.L.; Cook, N.J.; Gao, S.; Zhao, T.; Jiang, J. Constraints on Ore Genesis from Trace Ore Mineralogy: A New Occurrence of Kupčíkite and Paděraite from the Zhibula Cu Skarn Deposit, Southern Tibet. Minerals 2024, 14, 474. https://doi.org/10.3390/min14050474
Xu J, Ciobanu CL, Cook NJ, Gao S, Zhao T, Jiang J. Constraints on Ore Genesis from Trace Ore Mineralogy: A New Occurrence of Kupčíkite and Paděraite from the Zhibula Cu Skarn Deposit, Southern Tibet. Minerals. 2024; 14(5):474. https://doi.org/10.3390/min14050474
Chicago/Turabian StyleXu, Jing, Cristiana Liana Ciobanu, Nigel John Cook, Shen Gao, Taiping Zhao, and Jichen Jiang. 2024. "Constraints on Ore Genesis from Trace Ore Mineralogy: A New Occurrence of Kupčíkite and Paděraite from the Zhibula Cu Skarn Deposit, Southern Tibet" Minerals 14, no. 5: 474. https://doi.org/10.3390/min14050474