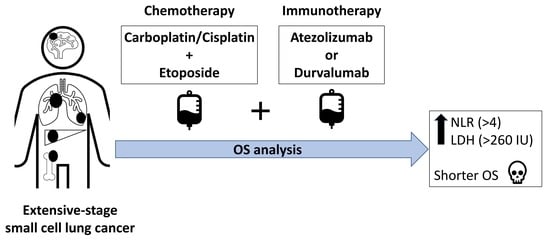
A Clinical Analysis of Anti-Programmed Death-Ligand 1 (PD-L1) Immune Checkpoint Inhibitor Treatments Combined with Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Treatment, and Follow-Up
2.2. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics and Clinical Information of the Study Patients
3.2. Efficacy of First-Line Anti-PD-L1 ICIs Combined with Chemotherapy
3.3. Analysis of Predictive Factors Associated with OS
3.4. First-Line-Treatment-Related Adverse Events (AEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Detterbeck, F.C.; Nicholson, A.G.; Franklin, W.A.; Marom, E.M.; Travis, W.D.; Girard, N.; Arenberg, D.A.; Bolejack, V.; Donington, J.S.; Mazzone, P.J.; et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J. Thorac. Oncol. 2016, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.E.; Zhang, X.; Fu, A.C.; Rams, A.R.; Baldasaro, J.A.; Ahmad, S.A.; Schlichting, M.; Marquis, P.; Benincasa, E.; Moulin, C.; et al. Development of a Conceptual Model of the Patient Experience in Small Cell Lung Cancer: A Qualitative Interview Study. Oncol. Ther. 2023, 11, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Li, J.M.; Yang, C.T. Forced Overexpression of Signal Transducer and Activator of Transcription 3 (STAT3) Activates Yes-Associated Protein (YAP) Expression and Increases the Invasion and Proliferation Abilities of Small Cell Lung Cancer (SCLC) Cells. Biomedicines 2022, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Petty, W.J.; Sands, J.M. An overview of lurbinectedin as a new second-line treatment option for small cell lung cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211020529. [Google Scholar] [CrossRef] [PubMed]
- Liguori, N.R.; Sanchez Sevilla Uruchurtu, A.; Zhang, L.; Abbas, A.E.; Lee, Y.S.; Zhou, L.; Azzoli, C.G.; El-Deiry, W.S. Preclinical studies with ONC201/TIC10 and lurbinectedin as a novel combination therapy in small cell lung cancer (SCLC). Am. J. Cancer Res. 2022, 12, 729–743. [Google Scholar]
- Tiseo, M.; Boni, L.; Ambrosio, F.; Camerini, A.; Baldini, E.; Cinieri, S.; Brighenti, M.; Zanelli, F.; Defraia, E.; Chiari, R.; et al. Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide With or Without Bevacizumab as First-Line Treatment in Extensive-Disease Small-Cell Lung Cancer: The GOIRC-AIFA FARM6PMFJM Trial. J. Clin. Oncol. 2017, 35, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Kuzevanova, A.; Apanovich, N.; Mansorunov, D.; Korotaeva, A.; Karpukhin, A. The Features of Checkpoint Receptor-Ligand Interaction in Cancer and the Therapeutic Effectiveness of Their Inhibition. Biomedicines 2022, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, N.; Bazhenova, L. New strategies in immunotherapy for lung cancer: Beyond PD-1/PD-L1. Ther. Adv. Respir. Dis. 2018, 12, 1753466618794133. [Google Scholar] [CrossRef]
- Hsu, J.C.; Nguyen, P.A.; Chen, Y.T.; Yang, S.C.; Lin, C.C.; Yang, Y.H.; Lin, Y.C.; Hsia, T.C.; Hsieh, H.C.; Wu, J.S.; et al. The Effectiveness and Safety of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Stage III/IV: A Multicenter Study. Front. Oncol. 2021, 11, 671127. [Google Scholar] [CrossRef]
- Liu, X.; Xing, H.; Liu, B. Current status and future perspectives of immune checkpoint inhibitors in extensive-stage small cell lung cancer. Am. J. Cancer Res. 2022, 12, 2447–2464. [Google Scholar] [PubMed]
- Arriola, E.; González-Cao, M.; Domine, M.; De Castro, J.; Cobo, M.; Bernabé, R.; Navarro, A.; Sullivan, I.; Trigo, J.M.; Mosquera, J.; et al. Addition of Immune Checkpoint Inhibitors to Chemotherapy vs Chemotherapy Alone as First-Line Treatment in Extensive-Stage Small-Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. Oncol. Ther. 2022, 10, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, H.; Li, C.; Wu, R.; Wang, Z.; Wang, Y.; Zhan, P.; Lv, T.; Zhang, F.; Song, Y.; et al. Efficacy and safety of first-line PD-1/PD-L1 inhibitor combinations for extensive-stage small-cell lung cancer: A Bayesian network meta-analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359231189430. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Ionova, Y.; Vuong, W.; Sandoval, O.; Fong, J.; Vu, V.; Zhong, L.; Wilson, L. Cost-Effectiveness Analysis of Atezolizumab Versus Durvalumab as First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer in the USA. Clin. Drug Investig. 2022, 42, 491–500. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, X.; Yi, L.; Zeng, X.; Tan, C. First-Line Chemo-Immunotherapy for Extensive-Stage Small-Cell Lung Cancer: A United States-Based Cost-Effectiveness Analysis. Front. Oncol. 2021, 11, 699781. [Google Scholar] [CrossRef]
- Lee, S.; Shim, H.S.; Ahn, B.C.; Lim, S.M.; Kim, H.R.; Cho, B.C.; Hong, M.H. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: A single-center experience. Cancer Immunol. Immunother. 2022, 71, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Kang, H.S.; Shin, A.Y.; Yeo, C.D.; Kim, S.K.; Kim, J.W.; Kim, S.J.; Lee, S.H. Investigation of poor predictive factors in extensive stage small cell lung cancer under etoposide-platinum-atezolizumab treatment. Thorac. Cancer 2022, 13, 3384–3392. [Google Scholar] [CrossRef]
- Chiang, C.L.; Yang, H.C.; Liao, Y.T.; Luo, Y.H.; Wu, Y.H.; Wu, H.M.; Chen, Y.M. Treatment and survival of patients with small cell lung cancer and brain metastasis. J. Neurooncol. 2023, 165, 343–351. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, F.; Xie, X.; Liu, T.; Zeng, C.; Chen, Z.; Zhou, M.; Deng, H.; Yang, Y.; Lin, X.; et al. A retrospective real-world experience of immunotherapy in patients with extensive stage small-cell lung cancer. Cancer Med. 2023, 12, 14881–14891. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Saito, G.; Fujimoto, D. Histologic transformation in lung cancer: When one door shuts, another opens. Ther. Adv. Med. Oncol. 2022, 14, 17588359221130503. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Xiang, Y.; Zhao, S.; Chen, J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol. Immunother. 2021, 70, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, J.A.; Timalsina, R.; Atmaca, A.; Rosery, V.; Frost, N.; Alt, J.; Waller, C.F.; Reinmuth, N.; Rohde, G.; Saalfeld, F.C.; et al. Clinical predictors of survival in patients with relapsed/refractory small-cell lung cancer treated with checkpoint inhibitors: A German multicentric real-world analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221097191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gong, X.; Sun, L.; Miao, L.; Zhou, Y. The Predictive Value of Pretreatment Lactate Dehydrogenase and Derived Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Meta-Analysis. Front. Oncol. 2022, 12, 791496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chou, C.H.; Su, H.H.; Tsai, Y.T.; Chiang, M.H.; Kuo, Y.J.; Chen, Y.P. Correlation between neutrophil-to-lymphocyte ratio and postoperative mortality in elderly patients with hip fracture: A meta-analysis. J. Orthop. Surg. Res. 2021, 16, 681. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Russo, D.; Maisto, M.; Troiano, G.; Caponio, V.C.A.; Annunziata, M.; Laino, L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral. Pathol. Med. 2022, 51, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, D.; Aufderstrasse, S.; König, L.; Adeberg, S.; Bozorgmehr, F.; Christopoulos, P.; Shafie, R.A.E.; Hörner-Rieber, J.; Kappes, J.; Thomas, M.; et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manag. Res. 2018, 10, 6563–6569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, L.; Qin, H.; Wang, W.J.; Ren, R.; Xu, M.D.; Chen, K.; Duan, W.; Gong, F.R.; Tao, M.; et al. Prognostic evaluation of patients with resectable lung cancer using systemic inflammatory response parameters. Oncol. Lett. 2019, 17, 2244–2256. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Crawford, J.; Herndon, D.; Gmitter, K.; Weiss, J. The impact of myelosuppression on quality of life of patients treated with chemotherapy. Future Oncol. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chi, X.; Shi, X.; Sun, Y.; Yang, X.; Wang, L.; Wang, B.; Li, H. Value of pretreatment serum lactate dehydrogenase as a prognostic and predictive factor for small-cell lung cancer patients treated with first-line platinum-containing chemotherapy. Thorac. Cancer 2021, 12, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Dawe, D.E.; Rittberg, R.; Syed, I.; Shanahan, M.K.; Moldaver, D.; Bucher, O.; Galloway, K.; Reynolds, K.; Paul, J.T.; Harlos, C.; et al. Real-world predictors of survival in patients with extensive-stage small-cell lung cancer in Manitoba, Canada: A retrospective cohort study. Front. Oncol. 2023, 13, 1191855. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Azzali, I.; Bittoni, A.; Marisi, G.; Rebuzzi, F.; Molinari, C.; Bartolini, G.; Matteucci, L.; Sullo, F.G.; Debonis, S.A.; et al. Inflammatory indices as prognostic markers in metastatic colorectal cancer patients treated with chemotherapy plus Bevacizumab. Ther. Adv. Med. Oncol. 2023, 15, 17588359231212184. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liang, Y.; Xue, S.; Yao, N.; Wei, L.; Cao, X.; Li, H.; Si, H.; Cao, J. Prognostic ability of lung immune prognostic index in limited-stage small cell lung cancer. BMC Cancer 2022, 22, 1233. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y.; Dong, J.; Wang, H.; Zhao, T.; Wang, X.; Liu, J.; Gao, S.; Shi, L.; Yang, S.; et al. BZW1 Facilitates Glycolysis and Promotes Tumor Growth in Pancreatic Ductal Adenocarcinoma Through Potentiating eIF2α Phosphorylation. Gastroenterology 2022, 162, 1256–1271.e14. [Google Scholar] [CrossRef]
- Belluomini, L.; Fiorica, F.; Frassoldati, A. Immune Checkpoint Inhibitors and Radiotherapy in NSCLC Patients: Not Just a Fluke. Oncol. Ther. 2019, 7, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chi, A. Combining stereotactic body radiotherapy with immunotherapy in stage IV non-small cell lung cancer. Front. Oncol. 2023, 13, 1211815. [Google Scholar] [CrossRef]
- Mei, T.; Xiu, W.; Yang, X.; Tian, X.; Yu, Y.; Xu, Y.; Zhou, L.; Zhou, X.; Liu, Y.; Zou, B.; et al. Development and validation of a nomogram for assessing survival in extensive-stage small-cell lung cancer patients with superior vena cava syndrome referred for thoracic radiotherapy: A comparison of upfront vs. consolidative approaches. Strahlenther. Onkol. 2021, 197, 1072–1083. [Google Scholar] [CrossRef]
- Petereit, C.; Zaba, O.; Teber, I.; Lüders, H.; Grohé, C. A rapid and efficient way to manage hyponatremia in patients with SIADH and small cell lung cancer: Treatment with tolvaptan. BMC Pulm. Med. 2013, 13, 55. [Google Scholar] [CrossRef]
- Berardi, R.; Mastroianni, C.; Lo Russo, G.; Buosi, R.; Santini, D.; Montanino, A.; Carnaghi, C.; Tiseo, M.; Chiari, R.; Camerini, A.; et al. Syndrome of inappropriate anti-diuretic hormone secretion in cancer patients: Results of the first multicenter Italian study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919877725. [Google Scholar] [CrossRef]
- Hsu, P.C.; Li, S.H.; Yang, C.T. Recurrent Pneumonitis Induced by Atezolizumab (Anti-Programmed Death Ligand 1) in NSCLC Patients Who Previously Experienced Anti-Programmed Death 1 Immunotherapy-Related Pneumonitis. J. Thorac. Oncol. 2018, 13, e227–e230. [Google Scholar] [CrossRef]
- Tyczynski, J.E.; Potluri, R.; Kilpatrick, R.; Mazumder, D.; Ghosh, A.; Liede, A. Incidence and Risk Factors of Pneumonitis in Patients with Non-Small Cell Lung Cancer: An Observational Analysis of Real-World Data. Oncol. Ther. 2021, 9, 471–488. [Google Scholar] [CrossRef]
- Sosa, A.; Lopez Cadena, E.; Simon Olive, C.; Karachaliou, N.; Rosell, R. Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol. 2018, 10, 1758835918764628. [Google Scholar] [CrossRef]



| Total | N = 72 (%) |
|---|---|
| Male | 69 (95.8%) |
| Female | 3 (4.2%) |
| Age year (median/range) | 65 (39 – 86) |
| ECOG PS | |
| 0–1 | 67 (93.1%) |
| 2 | 5 (6.9%) |
| BMI (median/range) | 23.81 (18.62–31.33) |
| Smoking status | |
| Non-smoker | 1 (1.4%) |
| Former/current smoker | 71(98.6%) |
| Histology | |
| Small-cell carcinoma | 72 (100%) |
| Stage | |
| IV | 72 (100%) |
| Neutrophil-to-lymphocyte ratio (NLR) (median/range) | 3.3 (1.3–49.0) |
| LDH (UL) (median/range) | 227.0 (150.0–2826.0) |
| Na (meq/L) (median/range) | 136.0 (115–149) |
| Na < 130 meq/L | 12 (16.7%) |
| Distant metastatic sites | |
| Contralateral lung | 26 (36.1%) |
| Pleura | 28 (38.9.1%) |
| Adrenal gland | 5 (6.9%) |
| Brain metastasis | 11 (15.3%) |
| Bone metastasis | 22 (30.6%) |
| Liver metastasis | 15 (20.8%) |
| Anti-PD-L1 inhibitors | |
| Atezolizumab | 21 (29.2%) |
| Durvalumab | 51 (70.8%) |
| Cycles of IO (median/range) | 8 (2–29) |
| Chemotherapy regimens | |
| Cisplatin + etoposide | 49 (68.1%) |
| Carboplatin + etoposide | 23 (31.9%) |
| Local radiation during IO + chemotherapy | |
| Brain | 11 (15.3%) |
| Bone | 5 (8.3%) |
| Prophylactic cranial irradiation (PCI) after chemotherapy | 4 (5.6%) |
| Variables | Patients N (%) | Median OS (Months) | Univariate Analysis p-Value HR (95% CI) | Multivariate Analysis | |
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | ||||
| Age | |||||
| ≤65 years | 38 | 15.40 | 0.763 | ||
| >65 years | 34 | 16.07 | 0.813 (0.462–1.432) | ||
| Sex | |||||
| Male | 69 | 16.07 | 0.01 | ||
| Female | 3 | 4.83 | 0.10 (0.027–0.370) | ||
| ECOG PS | |||||
| 0–1 | 67 | 16.07 | 0.075 | ||
| 2 | 5 | 8.97 | 2.337 (0.917–5.959) | ||
| NLR | |||||
| ≦4 | 46 | 17.33 | <0.001 | 1 | |
| >4 | 26 | 5.20 | 4.084 (2.291–7.283) | 2.994 (1.607–5.578) | 0.001 |
| LDH (UL) | |||||
| ≦260 | 41 | 20.4 | <0.001 | 1 | 0.001 |
| >260 | 31 | 5.8 | 3.802 (2.159–7.283) | 2.921 (1.591–5.362) | |
| Neutropenia | |||||
| Without grade 3 | 56 | 16.4 | 0.008 | ||
| With grade 3 | 16 | 5.20 | 2.52 (1.337–4.739) | ||
| Anti-PD-L1 inhibitors | |||||
| Atezolizumab | 21 | 15.4 | 0.115 | ||
| Durvalumab | 51 | 16.4 | 0.618 (0.344–1.108) | ||
| Metastatic sites | |||||
| Brain | |||||
| With brain metastasis | 11 | 16.1 | 0.713 | ||
| Without brain metastasis | 61 | 16.4 | 1.140 (0.568–2.287) | ||
| Bone | |||||
| With bone metastasis | 22 | 15.4 | 0.099 | ||
| Without bone metastasis | 50 | 16.1 | 1.630 (0.922–2.883) | ||
| Liver | |||||
| With liver metastasis | 15 | 15.4 | 0.606 | ||
| Without liver metastasis | 57 | 16.7 | 1.179 (0.631–2.201) | ||
| Adverse Events (AEs) | All n = 72 | Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Non-hematological | ||||
| Nausea or vomiting | 48 (66.7%) | 48 (66.7%) | 0 (0%) | 0 (0%) |
| Diarrhea | 13 (18.1%) | 12 (16.7%) | 1 (1.4%) | 0 (0%) |
| Constipation | 2 (2.8%) | 2 (2.8%) | 0 (0%) | 0 (0%) |
| Stomatitis | 10 (13.9%) | 10 (13.9%) | 0 (0%) | 0 (0%) |
| Fatigue | 26 (36.1%) | 26 (36.1%) | 0 (0%) | 0 (0%) |
| Skin rashes | 12 (16.7%) | 12 (16.7%) | 0 (0%) | 0 (0%) |
| Hair loss | 39 (54.2) | 39 (54.2%) | 0 (0%) | 0 (0%) |
| Increased liver transaminases | ||||
| Increased AST | 11 (15.3%) | 11 (15.3%) | 0 (0%) | 0 (0%) |
| Increased ALT | 12 (16.7%) | 12 (16.7%) | 0 (0%) | 0 (0%) |
| Increased creatinine | 13 (18.1%) | 12 (16.7%) | 1 (1.4%) | 0 (0%) |
| Hematological | ||||
| Neutropenia | 58 (80.5%) | 42 (58.3%) | 16 (22.2%) | 0 (0%) |
| Anemia | 59 (81.9%) | 46 (63.9%) | 13 (18.0%) | 0 (0%) |
| Thrombocytopenia | 47 (65.2%) | 41 (56.9%) | 6 (8.3%) | 0 (0%) |
| Any grade | ||||
| Febrile neutropenia | 22 (30.5%) | |||
| Study Name | Hsu et al. | IMpower133 [14] | CASPIAN [15] |
|---|---|---|---|
| Ant-PD-L1 ICIs | Atezolizumab/ durvalumab | Atezolizumab | Durvalumab |
| Chemotherapy regimens | Cis/carbo + etoposide | Carbo + etoposide | Cis/carbo + etoposide |
| Chemotherapy cycles | 4–6 | 4 cycles for all arms | 4 cycles for IO arms |
| Brain metastasis | 15.3% | 8.6% | 10.2% |
| Liver metastasis | 20.8% | 37% | 39.5% |
| ORR, % IO + chemotherapy | 59.7% | 60.2% | 68.0% |
| Median PFS, months | 6.63 | 5.2 | 5.1 |
| Median OS, months | 16.07 | 12.3 | 13.0 |
| >Grade 3 AE, % (any) | 51.3% | 57.1% | 52% |
| Treatment-related death | 0 | 1.5% | 2.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-C.; Wu, B.-C.; Wang, C.-C.; Chiu, L.-C.; Chang, C.-H.; Liu, P.-C.; Wu, C.-E.; Kuo, S.C.-H.; Ju, J.-S.; Huang, A.C.-C.; et al. A Clinical Analysis of Anti-Programmed Death-Ligand 1 (PD-L1) Immune Checkpoint Inhibitor Treatments Combined with Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer. Vaccines 2024, 12, 474. https://doi.org/10.3390/vaccines12050474
Hsu P-C, Wu B-C, Wang C-C, Chiu L-C, Chang C-H, Liu P-C, Wu C-E, Kuo SC-H, Ju J-S, Huang AC-C, et al. A Clinical Analysis of Anti-Programmed Death-Ligand 1 (PD-L1) Immune Checkpoint Inhibitor Treatments Combined with Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer. Vaccines. 2024; 12(5):474. https://doi.org/10.3390/vaccines12050474
Chicago/Turabian StyleHsu, Ping-Chih, Bing-Chen Wu, Chin-Chou Wang, Li-Chung Chiu, Chiung-Hsin Chang, Ping-Chi Liu, Chiao-En Wu, Scott Chih-Hsi Kuo, Jia-Shiuan Ju, Allen Chung-Cheng Huang, and et al. 2024. "A Clinical Analysis of Anti-Programmed Death-Ligand 1 (PD-L1) Immune Checkpoint Inhibitor Treatments Combined with Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer" Vaccines 12, no. 5: 474. https://doi.org/10.3390/vaccines12050474