Pediatric Type 1 Diabetes: Is Age at Onset a Determining Factor in Advanced Hybrid Closed-Loop Insulin Therapy?
Abstract
:1. Introduction
2. Materials and Methods
- -
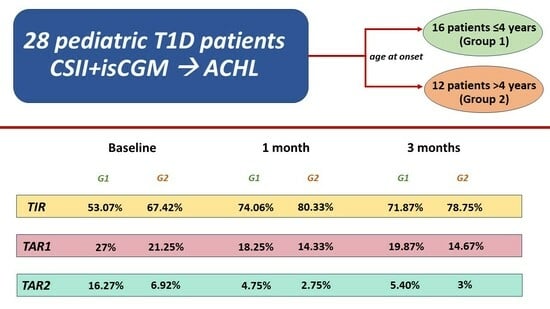
- Time in Range (TIR): percentage of time in which interstitial blood glucose levels are between 70 and 180 mg/dL.
- -
- Time Above Range 1 (TAR1): percentage of time in which interstitial blood glucose levels are between 180 and 250 mg/dL.
- -
- Time Above Range 2 (TAR2): percentage of time in which interstitial blood glucose is above 250 mg/dL.
- -
- Time Below Range 1 (TBR1): percentage of time in which interstitial blood glucose levels are between 70 and 54 mg/dL.
- -
- Time Below Range 2 (TBR2): percentage of time in which interstitial blood glucose is below 54 mg/dL.
- Pittsburgh Sleep Quality Index (PSQI): The sum of the scores of the 19 questions, or the total score, indicates the overall sleep quality of the person being evaluated. This total score can range from 0 to 21 points. The higher the total score, the worse the sleep quality. Thus, a total score less than or equal to five on the Pittsburgh scale indicates that, in general, sleep quality is optimal, while a total score greater than five suggests sleep disturbances, of greater or lesser severity [15].
- Diabetes Treatment Satisfaction Questionnaire (DTSQ) index: assesses satisfaction (status version, DTSQ-s) and change in satisfaction (change version, DTSQ-c) with treatment for T1D. DTSQ-s scores range from 0 to 48, and DTSQ-c scores range from −24 to +24, with higher scores indicating greater satisfaction [16].
3. Results
3.1. Study Population Characteristics
3.2. System Usability
3.3. Glycemic Outcomes
3.4. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Range of Risk Factor Levels: Control, Mortality, and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2017, 135, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.Y. Age of onset of diabetes and all-cause mortality. World J. Diabetes. 2020, 11, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Reusch, J.E.B. Cardiovascular function/dysfunction in adolescents with type 1 diabetes. Curr. Diab. Rep. 2011, 11, 185–192. [Google Scholar] [CrossRef]
- Vergès, B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020, 46, 442–449. [Google Scholar] [CrossRef]
- Karakuş, K.E.; Mutlu, G.Y.; Gökçe, T.; Eviz, E.; Can, E.; Muradoğlu, S.; Hatun, Ş. Insulin Requirements for Basal and Auto-Correction Insulin Delivery in Advanced Hybrid Closed-Loop System: 4193 Days’ Real-World Data of Children in Two Different Age Groups. J. Diabetes Sci. Technol. 2022, 19322968221106190. [Google Scholar] [CrossRef]
- Beato-Víbora, P.I.; Gallego-Gamero, F.; Ambrojo-López, A. Real-world outcomes with different technology modalities in type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, J.; Mathieu, C.; Aanstoot, H.-J.; Arrieta, A.; Da Silva, J.; Shin, J.; Cohen, O. Predictors of time in target glucose range in real-world users of the MiniMed 780G system. Diabetes Obes. Metab. 2022, 24, 2212–2221. [Google Scholar] [CrossRef]
- Reddy, M.; Jugnee, N.; Anantharaja, S.; Oliver, N. Switching from Flash Glucose Monitoring to Continuous Glucose Monitoring on Hypoglycemia in Adults with Type 1 Diabetes at High Hypoglycemia Risk: The Extension Phase of the I HART CGM Study. Diabetes Technol. Ther. 2018, 20, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.; Wright, N.P.; Yardley, D.; Campbell, F.; Randell, T.; Trevelyan, N.; Ghatak, A.; Hindmarsh, P.C. Real world use of hybrid-closed loop in children and young people with type 1 diabetes mellitus-a National Health Service pilot initiative in England. Diabet. Med. 2023, 40, e15015. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Lepore, G.; Battelino, T.; Arrieta, A.; Castañeda, J.; Grossman, B.; Shin, J.; Cohen, O. Real-World Performance of the MiniMedTM 780G System: First Report of Outcomes from 4120 Users. Diabetes Technol. Ther. 2022, 24, 113–119. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.-L.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Gomis, R.; Herrera-Pombo, J.L.; Calderón, A.; Rubio-Terrés, C.; Sarasa, P. Validación del cuestionario “Diabetes treatment satisfaction questionnaire” (DTSQ) en la población española. Pharm. Econ. Span. Res Artic. 2006, 3, 7–18. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Fernández, C.A.; Cardona-Hernandez, R.; Lozano, M.F.; Bahíllo-Curieses, P.; Arroyo-Díez, J.; León, M.C.; Martín-Frías, M.; Barreiro, S.C.; Delgado, A.M.; et al. Increased Presentation of Diabetic Ketoacidosis and Changes in Age and Month of Type 1 Diabetes at Onset during the COVID-19 Pandemic in Spain. J. Clin. Med. 2022, 11, 4338. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, A.; Battelino, T.; Scaramuzza, A.E.; Da Silva, J.; Castañeda, J.; Cordero, T.L.; Shin, J.; Cohen, O. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidence from 12 870 real-world users. Diabetes Obes. Metab. 2022, 24, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Lendínez-Jurado, A.; Gómez-Perea, A.; Ariza-Jiménez, A.B.; Tapia-Ceballos, L.; Becerra-Paz, I.; Martos-Lirio, M.F.; Moreno-Jabato, F.; Leiva-Gea, I. Impact on glucometric variables and quality of life of the advanced hybrid closed-loop system in pediatric and adolescent type 1 diabetes. J. Diabetes 2023, 15, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Tornese, G.; Buzzurro, F.; Carletti, C.; Faleschini, E.; Barbi, E. Six-Month Effectiveness of Advanced vs. Standard Hybrid Closed-Loop System in Children and Adolescents with Type 1 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 766314. [Google Scholar] [CrossRef] [PubMed]
- Piccini, B.; Pessina, B.; Casalini, E.; Lenzi, L.; Toni, S. Long-term effectiveness of advanced hybrid closed loop in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2022, 23, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gitelman, S.; DiMeglio, L.A.; Boulware, D.; Greenbaum, C.J. Fall in C-Peptide During First 4 Years from Diagnosis of Type 1 Diabetes: Variable Relation to Age, HbA1c, and Insulin Dose. Diabetes Care 2016, 39, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.J.; Inshaw, J.R.J.; Flaxman, C.S.; Leete, P.; Wyatt, R.C.; Russell, L.A.; Palmer, M.; Prasolov, D.; Worthington, T.; Hull, B.; et al. Circulating C-Peptide Levels in Living Children and Young People and Pancreatic β-Cell Loss in Pancreas Donors Across Type 1 Diabetes Disease Duration. Diabetes 2022, 71, 1591–1596. [Google Scholar] [CrossRef]
- Maddaloni, E.; Bolli, G.B.; Frier, B.M.; Little, R.R.; Leslie, R.D.; Pozzilli, P.; Buzzetti, R. C-peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes Obes. Metab. 2022, 24, 1912–1926. [Google Scholar] [CrossRef] [PubMed]

| Baseline (CSII + isCGM) | p-Val | 1 Month (AHCL—Automatic Mode) | p-Val | 3 Months (AHCL—Automatic Mode) | p-Val | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (≤4 year) | Group 2 (>4 year) | Group 1 (≤4 year) | Group 2 (>4 year) | Group 1 (≤4 year) | Group 2 (>4 year) | ||||
| TIR (%) Mean ± SD | 53.07 ± 9.44 | 67.42 ± 8.71 | p < 0.0004 | 74.06 ± 6.37 | 80.33 ± 7.49 | p < 0.03 | 71.87 ± 6.58 | 78.75 ± 5.94 | p < 0.009 |
| TAR1 (%) Mean ± SD | 27 ± 6.21 | 21.25 ± 4.63 | p < 0.011 | 18.25 ± 4.54 | 14.33 ± 5.74 | p < 0.006 | 19.87 ± 5.15 | 14.67 ± 4.36 | p < 0.009 |
| TAR2 (%) Mean ± SD | 16.27 ± 9.14 | 6.92 ± 4.89 | p < 0.003 | 4.75 ± 2.67 | 2.75 ± 1.96 | p = 0.0307 | 5.40 ± 2.85 | 3 ± 2.45 | p < 0.027 |
| TBR1 (%) Median (IQR) | 3.07 (0.50–3.50) | 3.75 (2–5.25) | p = 0.6041 | 2.25 (1–3.25) | 2.08 (1–3) | p = 0.7915 | 2.27 (1–3) | 2.50 (2–3) | p = 0.6314 |
| TBR2 (%) Median (IQR) | 0.60 (0–0.50) | 0.67 (0–1) | p = 0.8913 | 0.69 (0–1) | 0.50 (0–1) | p = 0.6216 | 0.60 (0–1) | 0.58 (0–1) | p = 0.948 |
| Glucose (mg/dL) Median (IQR) | 177.47 (169.50–186.50) | 153 (143.75–159.50) | p < 0.003 | 144.81 (139–150) | 141.92 (127.75–149.50) | p = 0.6569 | 153.67 (138.50–156.50) | 139.17 (132–147.50) | p < 0.074 |
| GMI (%) Mean ± SD | 7.46 ± 0.47 | 6.99 ± 0.30 | p < 0.036 | 6.80 ± 0.21 | 6.60 ± 0.29 | p = 0.1709 | 6.87 ± 0.26 | 6.64 ± 0.27 | p = 0.1 |
| HbA1c (%) Mean ± SD | 7.34 ± 0.64 | 6.68 ± 0.55 | p < 0.011 | - | - | - | 6.94 ± 0.56 | 6.65 ± 0.70 | p = 0.5082 |
| CV glucose (%) Mean ± SD | 39.83 ± 5.54 | 38.03 ± 4.84 | p = 0.3741 | 36.61 ± 3.75 | 33.60 ± 4.06 | p = 0.0574 | 36.72 ± 3.46 | 34.11 ± 3.09 | p < 0.05 |
| Parameters of use | |||||||||
| Total daily insulin (U/kg/day) Median (IQR) | 0.87 (0.63–1.06) | 0.81 (0.64–1.01) | p < 0.7414 | 1.04 (0.80–1.22) | 0.93 (0.76–1.10) | p = 0.3653 | 1.08 (0.82–1.15) | 0.95 (0.78–1.06) | p = 0.8470 |
| Total basal insulin (%) Mean ± SD | 38.19 ± 11.22 | 40.83 ± 9.97 | p = 0.7298 | 39.50 ± 6.95 | 38.92 ± 5.38 | p = 0.424 | 38.93 ± 6.46 | 38.25 ± 6.14 | p = 0.8948 |
| Total bolus insulin (%) Mean ± SD | 61.81 ± 11.22 | 59.17 ± 9.97 | p = 0.7298 | 60.50 ± 6.95 | 61.08 ± 5.38 | p = 0.424 | 61.07 ± 6.46 | 61.75 ± 6.14 | p = 0.8948 |
| Total autocorrection insulin (%) Mean ± SD | - | - | - | 24.25 ± 7.57 | 19.67 ± 9.60 | p = 0.4344 | 25.53 ± 8.15 | 21.92 ± 7.75 | p = 0.6474 |
| Fingerstick BG/day Mean ± SD | 3.81 ± 2.30 | 1.76 ± 1.47 | p < 0.0451 | 0.70 ± 0.82 | 0.53 ± 0.33 | p = 0.4396 | 0.71 ± 0.84 | 0.58 ± 0.48 | p = 0.5116 |
| Sensor use (%) Median (IQR) | 89.27 (89.50–98.50) | 88.50 (88–97.25) | p = 0.5593 | 97.19 (96–98) | 96.75 (96.50–98) | p = 0.596 | 95.80 (96–98) | 96.50 (95.75–97.25) | p = 0.8229 |
| Time in Auto Mode (%) Median (IQR) | - | - | - | 99.06 (98.75–100) | 99.17 (98.75–100) | p = 0.8675 | 97.47 (98–100) | 98.58 (97.75–100) | p = 0.4948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lendínez-Jurado, A.; López-Siguero, J.P.; Gómez-Perea, A.; Ariza-Jiménez, A.B.; Becerra-Paz, I.; Tapia-Ceballos, L.; Cruces-Ponce, C.; Jiménez-Hinojosa, J.M.; Morcillo, S.; Leiva-Gea, I. Pediatric Type 1 Diabetes: Is Age at Onset a Determining Factor in Advanced Hybrid Closed-Loop Insulin Therapy? J. Clin. Med. 2023, 12, 6951. https://doi.org/10.3390/jcm12216951
Lendínez-Jurado A, López-Siguero JP, Gómez-Perea A, Ariza-Jiménez AB, Becerra-Paz I, Tapia-Ceballos L, Cruces-Ponce C, Jiménez-Hinojosa JM, Morcillo S, Leiva-Gea I. Pediatric Type 1 Diabetes: Is Age at Onset a Determining Factor in Advanced Hybrid Closed-Loop Insulin Therapy? Journal of Clinical Medicine. 2023; 12(21):6951. https://doi.org/10.3390/jcm12216951
Chicago/Turabian StyleLendínez-Jurado, Alfonso, Juan Pedro López-Siguero, Ana Gómez-Perea, Ana B. Ariza-Jiménez, Icía Becerra-Paz, Leopoldo Tapia-Ceballos, Carmen Cruces-Ponce, José Manuel Jiménez-Hinojosa, Sonsoles Morcillo, and Isabel Leiva-Gea. 2023. "Pediatric Type 1 Diabetes: Is Age at Onset a Determining Factor in Advanced Hybrid Closed-Loop Insulin Therapy?" Journal of Clinical Medicine 12, no. 21: 6951. https://doi.org/10.3390/jcm12216951