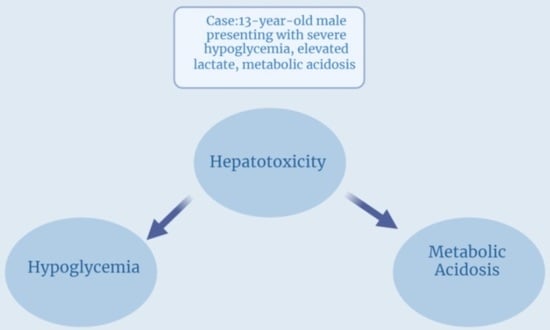
Profound Hypoglycemia and High Anion Gap Metabolic Acidosis in a Pediatric Leukemic Patient Receiving 6-Mercaptopurine
Abstract
:1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatia, S.; Landier, W.; Hageman, L.; Chen, Y.; Kim, H.; Sun, C.L.; Kornegay, N.; Evans, W.E.; Angiolillo, A.L.; Bostrom, B.; et al. Systemic Exposure to Thiopurines and Risk of Relapse in Children with Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. JAMA Oncol. 2015, 1, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Pinkel, D. Therapy of acute lymphoid leukemia in children. Leukemia 1992, 6 (Suppl. S2), 127–131. [Google Scholar] [PubMed]
- Bostrom, B.; Erdmann, G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am. J. Pediatr. Hematol. Oncol. 1993, 15, 80–86. [Google Scholar] [PubMed]
- Rosenfeld, E.; Getz, K.D.; Miller, T.P.; Seif, A.E.; Fisher, B.T.; Burrows, E.; Ramos, M.J.; De León, D.D.; Aplenc, R.; Morales, K.H.; et al. Incidence and risk factors for hypoglycemia during maintenance chemotherapy in pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2022, 69, e29467. [Google Scholar] [CrossRef] [PubMed]
- Melachuri, S.; Gandrud, L.; Bostrom, B. The association between fasting hypoglycemia and methylated mercaptopurine metabolites in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2014, 61, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Oliveira, J.; Sousa, C. Severe mercaptopurine-induced hypoglycemia in acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 2020, 37, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Devidas, M.; Cheng, S.C.; La, M.; Raetz, E.A.; Carroll, W.L.; Winick, N.J.; Hunger, S.P.; Gaynon, P.S.; Loh, M.L.; et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia 2008, 22, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Sobiak, J.; Skalska-Sadowska, J.; Chrzanowska, M.; Resztak, M.; Kołtan, S.; Wysocki, M.; Wachowiak, J. Thiopurine methyltransferase activity in children with acute myeloid leukemia. Oncol. Lett. 2018, 16, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.A.; Temkit, M.; Buras, M.R.; McLemore, R.Y.; Butler, R.K.; Chowdhury, Y.; Lipinski, C.A.; Traub, S.J. Serum Lactate and Mortality in Emergency Department Patients with Cancer. West J. Emerg. Med. 2018, 19, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.M.; Moon, J.E.; Lee, S.J.; Ko, C.W. Severe recurrent nocturnal hypoglycemia during chemotherapy with 6-mercaptopurine in a child with acute lymphoblastic leukemia. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Brackett, J.; Schafer, E.S.; Rau, R.E. Prevention of mercaptopurine-induced hypoglycemia using allopurinol to reduce methylated thiopurine metabolites. Pediatr. Blood Cancer 2019, 66, e27577. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Salo, M.K.; Mäkipernaa, A. Fasting hypoglycemia is common during maintenance therapy for childhood acute lymphoblastic leukemia. J. Pediatr. 2001, 138, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.; De Abreu, R.A.; Keizer-Garritsen, J.J.; Lambooy, L.H.; Ament, K.; ter Riet, P.G.; van Wering, E.R.; Trijbels, F.J.; Veerman, A.J.; Hoogerbrugge, P.M.; et al. Thiopurine methyltransferase in acute lymphoblastic leukaemia: Biochemical and molecular biological aspects. Eur. J. Cancer 2005, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bostrom, B. Allopurinol reverses mercaptopurine-induced hypoglycemia in patients with acute lymphoblastic leukemia. F1000Research 2019, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.Q.; Nguyen, M.; Zheng, L.; Ibanez, P.; Mei, L.; Kwan, L.Y.; Bradford, K.; Ting, C.; Targan, S.R.; Vasiliauskas, E.A. Split-dose administration of thiopurine drugs: A novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol. Ther. 2012, 36, 449–458. [Google Scholar] [CrossRef] [PubMed]


| Venous blood gas | pH 7.07 (nl 7.32–7.43), CO2 33 mmHg (nl 40–60 mmHg), bicarbonate 9 mmol/L (nl 22–27 mmol/L) |
| Platelet count | 50 × 109/L (nl 150–450 × 109/L) |
| Absolute neutrophil count | 320 × 109/L (nl 2500–6000 × 109/L) |
| Glucose | 30 mg/dL (nl 70–100 mg/dL) |
| Lactate | 8.7 mmol/L (nl < 2.0 mmol/L) |
| Beta hydroxybutyrate | 2.8 mmol/L (nl 0–0.5 mmol/L) |
| Aspartate aminotransaminase | 197 IU/L (nl 10–40 IU/L) |
| Alanine aminotransferase | 256 IU/L (nl 10–40 IU/L) |
| Total Bilirubin | 1.1 mg/dL (nl < 1.0 mgd/dL) |
| 6TGNs | 585 p/mol/8 × 108 RBC (nl 235–450 p/mol/8 × 108 RBC) |
| 6MMPNs | >52,320 pmol/8 × 108 RBC (nl ≤ 5700 pmol/8 × 108 RBC) |
| Date | 6-MMP Level |
|---|---|
| 3 November 2021 | >52,320 |
| 3 January 2022 | 1831 |
| 24 February 2022 | <422 |
| 4 November 2022 | 8768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Shea, M.; Kuhn, A.; Creo, A.L.; Kohorst, M.; Ferdjallah, A. Profound Hypoglycemia and High Anion Gap Metabolic Acidosis in a Pediatric Leukemic Patient Receiving 6-Mercaptopurine. Children 2024, 11, 160. https://doi.org/10.3390/children11020160
O’Shea M, Kuhn A, Creo AL, Kohorst M, Ferdjallah A. Profound Hypoglycemia and High Anion Gap Metabolic Acidosis in a Pediatric Leukemic Patient Receiving 6-Mercaptopurine. Children. 2024; 11(2):160. https://doi.org/10.3390/children11020160
Chicago/Turabian StyleO’Shea, Molly, Alexis Kuhn, Ana L. Creo, Mira Kohorst, and Asmaa Ferdjallah. 2024. "Profound Hypoglycemia and High Anion Gap Metabolic Acidosis in a Pediatric Leukemic Patient Receiving 6-Mercaptopurine" Children 11, no. 2: 160. https://doi.org/10.3390/children11020160