The Impact of Cr(III) and Cr(VI) on Lipid Accumulation in Chlorella pyrenoidosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cr Source Medium and Cultivation of Chlorella pyrenoidosa
2.2.1. Preparation of Cr Source Medium
2.2.2. Cultivation of Chlorella pyrenoidosa
2.3. Analysis of Chlorella pyrenoidosa
2.3.1. Analysis of Chlorella pyrenoidosa Lipid Content
2.3.2. Analysis of Chlorella pyrenoidosa Biomass
2.3.3. Analysis of Chlorella pyrenoidosa Enzyme Activity
2.3.4. Analysis of Cr(III) and Cr(VI)
2.3.5. Statistical Analysis
3. Results
3.1. Effects of Cr(III) and Cr(VI) on Biomass
3.2. Cr(III) and Cr(VI) on Effect of Lipid, Protein and Total Sugar Content
3.3. Chlorella pyrenoidosa of Cr(III) and Cr(VI) Stress Response
3.4. Distribution of Cr(III) and Cr(VI) in Algal Cells
3.5. The Effect of Cr (III) and Cr (VI) on the Activity of ACC, ME and PEPC
4. Discussion
4.1. Effects of Cr(III) and Cr(VI) on Biomass
4.2. Cr(III) and Cr(VI) on Effect of Lipid, Protein and Total Sugar Content
4.3. Chlorella pyrenoidosa of Cr(III) and Cr(VI) Stress Response
4.4. Distribution of Cr(III) and Cr(VI) in Algal Cells
4.5. Effect of Cr (III) and Cr (VI) on Activity of ACC, ME and PEPC
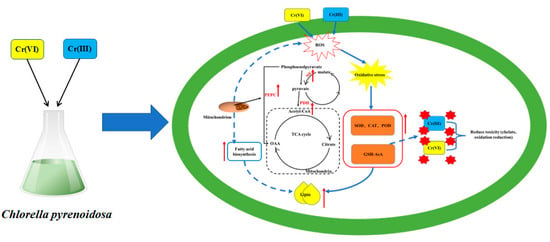
4.6. Mechanism of Influence of Cr(III) and Cr(VI) on Lipid Synthesis of Chlorella pyrenoidosa
5. Conclusions
- (1)
- When Cr(VI) concentration was in the range of 1.0–2.0 mg/L, it was beneficial to accumulate total sugar (37.28–48.33 mg/g FW > 32.83 mg/g FW in the control group). Concentrations of Cr(III) and Cr(VI) ranging from 0.5 to 1.0 mg/L were found to be conducive to lipid accumulation, with a weight percentage of 37.15–50.43 wt%, compared to the control group with 36.87 wt%. At a concentration of 0.5 mg/L of Cr(III), protein accumulation was observed to be higher (13.60 mg/g FW) compared to the control group (12.47 mg/g FW), indicating a beneficial effect.
- (2)
- Cr(VI) is 100 times more toxic than Cr(III), is easy to dissolve and has a strong oxidation capacity, changing the expression of genes and proteins. Microalgae cope with Cr(VI) stress by regulating the activity of antioxidant enzymes, such as increasing the activities of SOD, CAT and POD. Additionally, algal cells actively convert the more toxic Cr(VI) to Cr(III) through substances like GSH and AsA.
- (3)
- Cr(III) and Cr(VI) can help Chlorella pyrenoidosa improve the key enzyme of lipid synthesis in microalgae, but only under the conditions of 0.5–1.0 mg/L Cr(III) and Cr(VI), the lipid level of Chlorella pyrenoidosa is higher than that of the control group. Although the high Cr environment can help the key enzymes of lipid synthesis in microalgae maintain high activity to produce more lipids, the excessive production of reactive oxygen species induced by high Cr will also accelerate the destruction of the synthesized lipids and other macromolecules, which is unfavorable to the final result.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedayatkhah, A.; Cretoiu, M.S.; Emtiazi, G.; Stal, L.J.; Bolhuis, H. Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. J. Environ. Manag. 2018, 227, 313–320. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Sarker, S.S.; Akter, T.; Parveen, S.; Uddin, M.T.; Mondal, A.K.; Sujan, S.A. Microalgae-based green approach for effective chromium removal from tannery effluent: A review. Arab. J. Chem. 2023, 16, 105085. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Chandra, R.; Mishra, B.K. Potential human health hazard due to bioavailable heavy metal exposure via consumption of plants with ethnobotanical usage at the largest chromite mine of India. Environ. Geochem. Health 2020, 42, 4213–4231. [Google Scholar] [CrossRef]
- Luo, L.; Yang, C.; Jiang, X.; Guo, W.; Ngo, H.H.; Wang, X.C. Impacts of fulvic acid and Cr (VI) on metabolism and chromium removal pathways of green microalgae. J. Hazard. Mater. 2023, 459, 132171. [Google Scholar] [CrossRef]
- Shen, L.; Saky, S.A.; Yang, Z.; Ho, S.H.; Chen, C.; Qin, L.; Lu, Y. The critical utilization of active heterotrophic microalgae for bioremoval of Cr (VI) in organics co-contaminated wastewater. Chemosphere 2019, 228, 536–544. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- de Moura Sousa, L.; Moreira, F.S.; Cardoso, V.L.; Batista, F.R.X. Light intensity effect on the performance of Rhodobacter capsulatus in removal of chromium from effluent. J. Water Process. 2023, 52, 103567. [Google Scholar] [CrossRef]
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard. Mater. 2007, 146, 270–277. [Google Scholar] [CrossRef]
- Lanka, S.; Murari, S.G. Aquatic Plants in Phytoextraction of Hexavalent Chromium and Other Metals from Electroplating Effluents. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management: Principles, Monitoring and Remediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–139. [Google Scholar]
- Devi, A.; Verma, M.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Mulla, S.I.; Bharagava, R.N. Microalgae: A green eco-friendly agents for bioremediation of tannery wastewater with simultaneous production of value-added products. Chemosphere 2023, 336, 139192. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Aharchaou, I.; Rosabal, M.; Liu, F.; Battaglia, E.; Vignati, D.A.; Fortin, C. Bioaccumulation and subcellular partitioning of Cr (III) and Cr (VI) in the freshwater green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2017, 182, 49–57. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hasht, A.M.; Saratale, G.D.; Maiti, A.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef]
- Lu, M.M.; Gao, F.; Li, C.; Yang, H.L. Response of microalgae Chlorella vulgaris to Cr stress and continuous Cr removal in a membrane photobioreactor. Chemosphere 2021, 262, 128422. [Google Scholar] [CrossRef]
- Kafil, M.; Berninger, F.; Koutra, E.; Kornaros, M. Utilization of the microalga Scenedesmus quadricauda for hexavalent chromium bioremediation and biodiesel production. Bioresour. Technol. 2022, 346, 126665. [Google Scholar] [CrossRef]
- Fozer, D.; Kiss, B.; Lorincz, L.; Szekely, E.; Mizsey, P.; Nemeth, A. Improvement of microalgae biomass productivity and subsequent biogas yield of hydrothermal gasification via optimization of illumination. Renew. Energy 2019, 138, 1262–1272. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, J.; Gong, D.; Zhao, X.; Qi, Y.; Su, Y.; Ma, W. The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci. Total Environ. 2018, 633, 593–599. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Maurya, R.; Trivedi, K.; Patidar, S.K.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Kumar, S.S.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Microalgal consortia for municipal wastewater treatment–Lipid augmentation and fatty acid profiling for biodiesel production. J. Photochem. 2020, 202, 111638. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE 2014, 9, e112270. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Yang, Z.; Yang, W.; Zhou, J.; Cen, K. Microstructure and antioxidative capacity of the microalgae mutant Chlorella PY-ZU1 during tilmicosin removal from wastewater under 15% CO2. J. Hazard. Mater. 2017, 324, 414–419. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Branca, M.; Micera, G.; Kozlowski, H.; Swiatek, J. Reduction of chromate ions by glutathione tripeptide in the presence of sugar ligands. J. Inorg. 1990, 39, 217–226. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.F.; Kong, F.; Song, Q.; Ren, N.Q.; Ren, H.Y. Simultaneous chromium removal and lipid accumulation by microalgae under acidic and low temperature conditions for promising biodiesel production. Bioresour. Technol. 2023, 370, 128515. [Google Scholar] [CrossRef]
- Schiavon, M.; Galla, G.; Wirtz, M.; Pilon-Smits, E.A.; Telatin, V.; Quaggiotti, S.; Malagoli, M. Transcriptome profiling of genes differentially modulated by sulfur and chromium identifies potential targets for phytoremediation and reveals a complex S–Cr interplay on sulfate transport regulation in B. juncea. J. Hazard. Mater. 2012, 239, 192–205. [Google Scholar] [CrossRef]
- Kováčik, J.; Rotková, G.; Bujdoš, M.; Babula, P.; Peterková, V.; Matúš, P. Ascorbic acid protects Coccomyxa subellipsoidea against metal toxicity through modulation of ROS/NO balance and metal uptake. J. Hazard. Mater. 2017, 339, 200–207. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.W.; Hsu, C.Y.; Lee, L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; Suastes-Rivas, J.K. Sustainable biorefinery associated with wastewater treatment of Cr (III) using a native microalgae consortium. Fuel 2021, 290, 119040. [Google Scholar] [CrossRef]
- Li, N.; Qin, L.; Zhang, L.; Geng, W. Extracellular adsorption, intracellular accumulation and tolerance mechanisms of Cyclotella sp. to Cr (VI) stress. Chemosphere 2021, 270, 128662. [Google Scholar] [CrossRef]
- Ikaran, Z.; Suárez-Alvarez, S.; Urreta, I.; Castañón, S. The effect of nitrogen limitation on the physiology and metabolism of Chlorella vulgaris var L3. Algal Res. 2015, 10, 134–144. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nagano, Y. Plant acetyl-CoA carboxylase: Structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci. Biotechnol. Biochem. 2004, 68, 1175–1184. [Google Scholar] [CrossRef]
- Ke, J.; Wen, T.N.; Nikolau, B.J.; Wurtele, E.S. Coordinate regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase. Plant Physiol. 2000, 122, 1057–1072. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, T.; Han, B.; Dong, X.; Geng, S.; Ning, D.; Yu, X. Simultaneous enhancement of biomass and lipid production of Monoraphidium sp. QLZ-3 in a photobioreactor by using walnut shell extracts. Energy Convers. 2020, 204, 112326. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef]
- Han, W.; Ding, W.; Lu, S.; Song, K.; Chen, C.; Zhou, X. Effects of nutrient composition, lighting conditions, and metal ions on the growth and lipid yield of the high-lipid-yielding microalgae (Chlorella pyrenoidosa) cultivated in municipal wastewater. J. Environ. 2021, 9, 106491. [Google Scholar] [CrossRef]
- Ren, L.J.; Huang, H.; Lian, M.; Ji, X.J. Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess. Biosyst. Eng. 2009, 32, 837–843. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, B.; Ding, H.; Zhang, J.; Li, S. The role of NADP-malic enzyme in plants under stress. Plant Sci. 2019, 281, 206–212. [Google Scholar] [CrossRef]
- Seo, S.; Jeon, H.; Chang, K.S. Enhanced biomass production by Phaeodactylum tricornutum overexpressing phosphoenolpyruvate carboxylase. Algal Res. 2018, 31, 489–496. [Google Scholar] [CrossRef]
- Mamedov, T.G.; Moellering, E.R.; Chollet, R. Identification and expression analysis of two inorganic C-and N-responsive genes encoding novel and distinct molecular forms of eukaryotic phosphoenolpyruvate carboxylase in the green microalga Chlamydomonas reinhardtii. Plant J. 2005, 42, 832–843. [Google Scholar] [CrossRef]
- Moellering, E.R.; Ouyang, Y.; Mamedov, T.G.; Chollet, R. The two divergent PEP-carboxylase catalytic subunits in the green microalga Chlamydomonas reinhardtii respond reversibly to inorganic-N supply and co-exist in the high-molecular-mass, hetero-oligomeric Class-2 PEPC complex. FEBS Lett. 2007, 581, 4871–4876. [Google Scholar] [CrossRef]







| Reagents | Chemical Formula | Purity | Manufacturer | Location |
|---|---|---|---|---|
| Chromium trichloride hexahydrate | CrCl3·6H2O | ≥98.00% | Aladdin Reagent Co., Ltd. | Shanghai, China |
| Copper sulfate pentahydrate | CuSO4·5H2O | ≥99.00% | ||
| Sodium carbonate | Na2CO3 | ≥99.50% | ||
| Dipotassium phosphate | K2HPO4 | ≥99.00% | ||
| Ammonium ferric citrate | C6H11FeNO7 | ≥99.00%, Fe: 20.50–22.50% | ||
| Disodium dihydrate ethylenediamine tetraacetate | C10H14N2Na2O8·2H2O | ≥98.00% | ||
| Boric acid | H3BO3 | ≥99.00% | ||
| Zinc sulfate heptahydrate | ZnSO4·7H2O | ≥99.00% | ||
| Magnesium sulfate heptahydrate | MgSO4·7H2O | ≥99.00% | ||
| Sodium molybdate dihydrate | Na2MoO4·2H2O | ≥99.00% | ||
| Calcium chloride dihydrate | CaCl2·2H2O | ≥99.90% | Macklin Biochemical Technology Co., Ltd. | Shanghai, China |
| Manganese chloride tetrahydrate | MnCl2·4H2O | ≥99.00% | ||
| Cobalt nitrate hexahydrate | Co(NO3)2·6H2O | ≥99.00% | ||
| Sodium nitrate | NaNO3 | ≥99.00% | SHENTAI Chemical Industry Co., Ltd. | Tianjin, China |
| Potassium dichromate | K2Cr2O7 | ≥99.80% |
| Compositions | NaNO3 | K2HPO4 | MgSO4 7H2O | C6H8O7 | C6H11FeNO7 | Na2EDTA | CaCl2 2H2O | Na2CO3 | H3BO3 | MnCl4 4H2O | ZnSO4 7H2O | Na2MoO4 2H2O | CuSO4 5H2O | Co(NO3)2 6H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 1.5 | 0.04 | 0.75 | 0.006 | 0.006 | 0.001 | 0.036 | 0.02 | 0.00286 | 0.00182 | 0.00022 | 0.0004 | 0.00008 | 0.0005 |
| Living Cell | Dead Cell | Cell Crushing Supernatant | |
|---|---|---|---|
| Decrement rate (mg/L/day) | 3.73 | 1.52 | 0.38 |
| Specific reduction rate (mg/g/day) | 2.07 | 0.95 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Guo, H.; Yu, Q.; Wang, Y.; Liu, H.; Zeng, Y.; Wang, Y.; Liu, C.; Li, J. The Impact of Cr(III) and Cr(VI) on Lipid Accumulation in Chlorella pyrenoidosa. Processes 2024, 12, 905. https://doi.org/10.3390/pr12050905
Liu T, Guo H, Yu Q, Wang Y, Liu H, Zeng Y, Wang Y, Liu C, Li J. The Impact of Cr(III) and Cr(VI) on Lipid Accumulation in Chlorella pyrenoidosa. Processes. 2024; 12(5):905. https://doi.org/10.3390/pr12050905
Chicago/Turabian StyleLiu, Tianji, Huawei Guo, Qing Yu, Yajun Wang, Huan Liu, Yanan Zeng, Yitong Wang, Chunyu Liu, and Junguo Li. 2024. "The Impact of Cr(III) and Cr(VI) on Lipid Accumulation in Chlorella pyrenoidosa" Processes 12, no. 5: 905. https://doi.org/10.3390/pr12050905