Efficient Anthocyanin Recovery from Black Bean Hulls Using Eutectic Mixtures: A Sustainable Approach for Natural Dye Development
Abstract
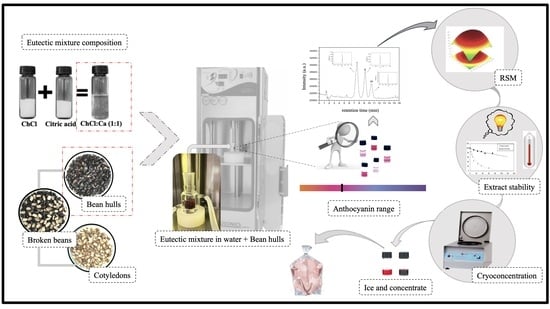
:1. Introduction
2. Materials and Methods
2.1. Sample and Chemicals
2.2. Sample Preparation
2.3. Optimization of the Ultrasound-Assisted Extractions (UAE)
2.4. Characterization of Anthocyanin-Rich Extracts
2.4.1. Total Monomeric Anthocyanin (TMA)
2.4.2. Total Phenolic Content (TPC)
2.4.3. In Vitro Antioxidant Activity (AA)
2.4.4. Identification and Quantification of Individual Anthocyanins
2.5. Stability of Anthocyanin-Rich Extracts
2.5.1. Impact of Temperature on Anthocyanin Degradation
Thermodynamics of the Degradative Process
2.5.2. Effect of Light on Anthocyanin Degradation
2.5.3. Block Cryoconcentration Assisted by Centrifugation
2.6. Green Metrics
2.7. UV-Vis Spectroscopy at Different pHs
2.8. Statistical Analysis
3. Results and Discussion
3.1. UAE Optimization
3.2. Individual Anthocyanins in Optimized Extract
3.3. Thermo and Photostability of Anthocyanin-Rich Extracts
3.4. Cryoconcentration of Anthocyanin-Rich Extracts
3.5. Green Metrics
3.6. Response of Anthocyanin Extract to pHs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Tapia, M.; Hernández-Velázquez, I.; Pichardo-Ontiveros, E.; Granados-Portillo, O.; Gálvez, A.; Tovar, A.R.; Torres, N. Consumption of Cooked Black Beans Stimulates a Cluster of Some Clostridia Class Bacteria Decreasing Inflammatory Response and Improving Insulin Sensitivity. Nutrients 2020, 12, 1182. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Suárez Valles, B.; Ferreira Fernández, J.J. Phenolic Content and Antioxidant Activity in Seeds of Common Bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef]
- Anino, C.; Onyango, A.N.; Imathiu, S.; Maina, J.; Onyangore, F. Chemical Composition of the Seed and ‘Milk’ of Three Common Bean (Phaseolus vulgaris L.) Varieties. J. Food Meas. Charact. 2019, 13, 1242–1249. [Google Scholar] [CrossRef]
- Cominelli, E.; Sparvoli, F.; Lisciani, S.; Forti, C.; Camilli, E.; Ferrari, M.; Le Donne, C.; Marconi, S.; Juan Vorster, B.; Botha, A.M.; et al. Antinutritional Factors, Nutritional Improvement, and Future Food Use of Common Beans: A Perspective. Front. Plant Sci. 2022, 13, 992169. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 23 January 2021).
- Andrade, E.T.D.; Corrêa, P.C.; Martins, J.H.; Alvarenga, E.M. Evaluation of Mechanical Damage in Edible Bean Seeds By Electrical Conductivity. Eng. Agrícola 1999, 3, 406–412. [Google Scholar] [CrossRef]
- Khazaei, J. Influence of Impact Velocity and Moisture on Mechanical Damage in Beans Influence of Impact Velocity and Moisture Content on Mechanical Damages of White Kidney Beans Under Loadings. Cercet. Agron. Mold. 2009, XLII, 5–18. [Google Scholar]
- Teixeira, R.F.; Benvenutti, L.; Burin, V.M.; Gomes, T.M.; Ferreira, S.R.S.; Zielinski, A.A.F. An Eco-Friendly Pressure Liquid Extraction Method to Recover Anthocyanins from Broken Black Bean Hulls. Innov. Food Sci. Emerg. Technol. 2021, 67, 102587. [Google Scholar] [CrossRef]
- Kuasnei, M.; Wojeicchowski, J.P.; Santos, N.H.; Pinto, V.Z.; Ferreira, S.R.S.; Zielinski, A.A.F. Modifiers Based on Deep Eutectic Mixtures: A Case Study for the Extraction of Anthocyanins from Black Bean Hulls Using High Pressure Fluid Technology. J. Supercrit. Fluids 2022, 191, 105761. [Google Scholar] [CrossRef]
- Khezerlou, A.; Tavassoli, M.; Alizadeh Sani, M.; Ehsani, A.; McClements, D.J. Smart Packaging for Food Spoilage Assessment Based on Hibiscus sabdariffa L. Anthocyanin-Loaded Chitosan Films. J. Compos. Sci. 2023, 7, 404. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring Some Colour to Your Package: Freshness Indicators Based on Anthocyanin Extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Doğan, M. Application of Deep Eutectic Solvents as a Green and Biodegradable Media for Extraction of Anthocyanin from Black Carrots. LWT-Food Sci. Technol. 2021, 138, 110775. [Google Scholar] [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep Eutectic Solvents (DESs) and Natural Deep Eutectic Solvents (NADESs): Designer Solvents for Green Extraction of Anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Perera, C.O.; Alzahrani, M.A.J. Ultrasound as a Pre-Treatment for Extraction of Bioactive Compounds and Food Safety: A Review. LWT 2021, 142, 111114. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents Providing Enhanced Stability of Natural Colorants from Safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as Green Extraction Solvent: Principles and Reasons for Its Use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Turan, O.; Isci, A.; Yılmaz, M.S.; Tolun, A.; Sakiyan, O. Microwave-Assisted Extraction of Pectin from Orange Peel Using Deep Eutectic Solvents. Sustain. Chem. Pharm. 2024, 37, 101352. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D. Cryoconcentration Technology in the Bio-Food Industry: Principles and Applications. LWT-Food Sci. Technol. 2009, 42, 679–685. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Pierre, L.; Pensaben, J.M. Protection of Polyphenols in Blueberry Juice by Vacuum-Assisted Block Freeze Concentration. Food Chem. Toxicol. 2017, 109, 1093–1102. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Zardo, D.M.; Alberti, A.; Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A. Effect of Cryoconcentration Process on Phenolic Compounds and Antioxidant Activity in Apple Juice. J. Sci. Food Agric. 2019, 99, 2786–2792. [Google Scholar] [CrossRef]
- Martín Carla, V.-S.; Bastías-Montes, J.M.; Villagra-Jorquera, C.; Salinas-Huenchulao, G.; Flores-Ríos, A.; Gonzáles-Díaz, N.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; Quevedo-León, R. Effect of cryoconcentration assisted by centrifugation-filtration on bioactive compounds and microbiological quality of aqueous maqui (Aristotelia chilensis (mol.) stuntz) and calafate (Berberis microphylla g. forst) extracts pretreated with high-pressure homogenization. Processes 2021, 9, 692. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. The Influence of Chemical Structure and the Presence of Ascorbic Acid on Anthocyanins Stability and Spectral Properties in Purified Model Systems. Foods 2019, 8, 207. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Murean, C.; Râpeanu, G.; Croitoru, C. Journal of Food Quality Thermal Degradation of Plum Anthocyanins: Comparison of Kinetics from Simple to Natural Systems. J. Food Qual. 2018, 2018, 1598756. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Handb. Food Anal. Chem. 2001, 2, 19–31. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolic with Phosphomolibdic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Development and Characterisation of Carbon Nanotube-Reinforced Polyurethane Foams. EMPA Act. 1999, 26, 51. [Google Scholar] [CrossRef]
- Peron, D.V.; Fraga, S.; Antelo, F. Thermal Degradation Kinetics of Anthocyanins Extracted from Juçara (Euterpe edulis Martius) and “Italia” Grapes (Vitis vinifera L.), and the Effect of Heating on the Antioxidant Capacity. Food Chem. 2017, 232, 836–840. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana, P.; Moreno, J.; Cerda, E.; Parra, P. Vacuum-Assisted Block Freeze Concentration Applied to Wine. Innov. Food Sci. Emerg. Technol. 2016, 36, 330–335. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, P.; Chen, P.; Wu, D. Highly Efficient Enzymatic Conversion of Rutin to Isoquercitrin and l-Rhamnose Using Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 14905–14913. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.V.; Boiteux, J.; Silva, M.F. Green Analytical Chemistry Metrics: Towards a Sustainable Phenolics Extraction from Medicinal Plants. Microchem. J. 2018, 141, 438–443. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a Semi-Quantitative Tool to Select an Organic Preparation Based on Economical and Ecological Parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of Bioactive Compounds from Beet Leaves through Simultaneous Extraction: Modelling and Process Optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Fernández-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; García-Barroso, C. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Jabuticaba (Myrciaria cauliflora) Fruit through a Box-Behnken Experimental Design. Food Sci. Technol. 2019, 39, 1018–1029. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of Phenolic Compounds from Cocoa Shell: Modeling Using Response Surface Methodology and Artificial Neural Networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Khajeh, M. Response Surface Modelling of Lead Pre-Concentration from Food Samples by Miniaturised Homogenous Liquid-Liquid Solvent Extraction: Box-Behnken Design. Food Chem. 2011, 129, 1832–1838. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Singh, V. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Zou, T.B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Mulberry, Using Response Surface Methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Q.; Belwal, T.; Li, L.; Aalim, H.; Wu, Q.; Duan, Z.; Zhang, X.; Luo, Z. Ultrasonic Impact on Viscosity and Extraction Efficiency of Polyethylene Glycol: A Greener Approach for Anthocyanins Recovery from Purple Sweet Potato. Food Chem. 2019, 283, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ping-Kou; Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Sanchez-Camargo, A.d.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria cauliflora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Ding, C.; Yang, R.; Jiang, L. Highly Efficient Extraction of Mulberry Anthocyanins in Deep Eutectic Solvents: Insights of Degradation Kinetics and Stability Evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black Bean Anthocyanin-Rich Extracts as Food Colorants: Physicochemical Stability and Antidiabetes Potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of Malvidin, Cyanidin and Delphinidin on Human Adipose Mesenchymal Stem Cell Differentiation into Adipocytes, Chondrocytes and Osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Cvorovic, J.; Tramer, F.; Granzotto, M.; Candussio, L.; Decorti, G.; Passamonti, S. Oxidative Stress-Based Cytotoxicity of Delphinidin and Cyanidin in Colon Cancer Cells. Arch. Biochem. Biophys. 2010, 501, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Wu, G.; Han, D.; Stipkovits, L.; Wu, X.; Tang, S.; Brennan, M.A.; Brennan, C.S. The Effects of Bioactive Compounds from Blueberry and Blackcurrant Powders on the Inhibitory Activities of Oat Bran Pastes against α-Amylase and α-Glucosidase Linked to Type 2 Diabetes. Food Res. Int. 2020, 138, 109756. [Google Scholar] [CrossRef]
- Sapian, S.; Taib, I.S.; Katas, H.; Latip, J.; Zainalabidin, S.; Hamid, Z.A.; Anuar, N.N.M.; Budin, S.B. The Role of Anthocyanin in Modulating Diabetic Cardiovascular Disease and Its Potential to Be Developed as a Nutraceutical. Pharmaceuticals 2022, 15, 1344. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural Deep Eutectic Solvent Enhanced Pulse-Ultrasonication Assisted Extraction as a Multi-Stability Protective and Efficient Green Strategy to Extract Anthocyanin from Blueberry Pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus Roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanins in Acerola Pulp: Comparison between Ohmic and Conventional Heat Treatment. Food Chem. 2013, 136, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-Isothermal Kinetics of Thermal Degradation of Chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef]
- Molaeafard, S.; Jamei, R.; Poursattar Marjani, A. Co-Pigmentation of Anthocyanins Extracted from Sour Cherry (Prunus cerasus L.) with Some Organic Acids: Color Intensity, Thermal Stability, and Thermodynamic Parameters. Food Chem. 2021, 339, 128070. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block Freeze Concentration Assisted by Centrifugation Applied to Blueberry and Pineapple Juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an Intelligent Film Based on Chitosan/Oxidized Chitin Nanocrystals Incorporating Black Rice Bran Anthocyanins for Seafood Spoilage Monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]




| Essay | x1 | x2 | x3 | TMA | R2 | R2adj | TPC | R2 | R2adj | DPPH | R2 | R2adj | ABTS | R2 | R2adj |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 150 | 9 | 3.7 ± 0.2 | 0.95 | 0.92 | 73 ± 4 | 0.81 | 0.77 | 389 ± 2 | 0.87 | 0.85 | 707 ± 4 | 0.94 | 0.93 |
| 2 | 70 | 450 | 5 | 4.1 ± 0.4 | 105 ± 1 | 417 ± 9 | 966 ± 7 | ||||||||
| 3 | 50 | 150 | 1 | 2.8 ± 0.1 | 47 ± 3 | 276 ± 6 | 543 ± 6 | ||||||||
| 4 | 30 | 300 | 1 | 1.6 ± 0.1 | 37 ± 7 | 253 ± 11 | 373 ± 3 | ||||||||
| 5 | 70 | 300 | 9 | 3.9 ± 0.1 | 112 ± 10 | 434 ± 2 | 893 ± 5 | ||||||||
| 6 | 50 | 300 | 5 | 3.7 ± 0.1 | 74 ± 0.5 | 334 ± 2 | 728 ± 8 | ||||||||
| 7 | 50 | 450 | 1 | 3.2 ± 0.2 | 44 ± 6 | 276 ± 3 | 671 ± 4 | ||||||||
| 8 | 30 | 300 | 9 | 3.1 ± 0.1 | 49 ± 1 | 274 ± 7 | 628 ± 3 | ||||||||
| 9 | 50 | 300 | 5 | 3.7 ± 0.2 | 63 ± 9 | 340 ± 3 | 659 ± 3 | ||||||||
| 10 | 70 | 150 | 5 | 3.9 ± 0.1 | 92 ± 5 | 350 ± 5 | 863 ± 7 | ||||||||
| 11 | 50 | 300 | 5 | 3.7 ± 0.2 | 83 ± 3 | 345 ± 12 | 738 ± 5 | ||||||||
| 12 | 70 | 300 | 1 | 3.5 ± 0.3 | 83 ± 9 | 328 ± 5 | 872 ± 12 | ||||||||
| 13 | 50 | 450 | 9 | 3.9 ± 0.1 | 90 ± 8 | 349 ± 8 | 801 ± 13 | ||||||||
| 14 | 30 | 450 | 5 | 3.0 ± 0.2 | 55 ± 0.4 | 255 ± 8 | 522 ± 8 | ||||||||
| 15 | 30 | 150 | 5 | 2.5 ± 0.1 | 68 ± 6 | 248 ± 5 | 480 ± 11 |
| Extract | T (°C) | R2 | Q10 | ∆H (kJ mol−1) | ∆G (kJ mol−1) | ∆S (J mol−1 K−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| 8.2% of eutectic mixture added in water | 60 | 0.0004 | 0.89 | 28.88 | 95.94 | 2.50 | 84.6 | 195.2 | −332.1 |
| 70 | 0.001 | 0.95 | 11.55 | 38.38 | 3.00 | 84.5 | 198.6 | −332.4 | |
| 80 | 0.003 | 0.96 | 3.85 | 12.79 | 1.67 | 84.4 | 201.2 | −330.7 | |
| 90 | 0.005 | 0.8 | 2.31 | 7.68 | 84.4 | 205.4 | −333.5 | ||
| Water | 60 | 0.005 | 0.99 | 2.31 | 7.68 | 1.40 | 27.3 | 188.2 | −483.3 |
| 70 | 0.007 | 0.92 | 1.65 | 5.48 | 1.14 | 27.2 | 193.0 | −483.4 | |
| 80 | 0.008 | 0.9 | 1.44 | 4.80 | 1.63 | 27.1 | 198.3 | −485.0 | |
| 90 | 0.013 | 0.84 | 0.89 | 2.95 | 27.0 | 202.6 | −483.5 |
| Extract and Cryoconcentration Cycle | Anthocyanins in Concentrate (g.mL−1) | Anthocyanins in Ice (g mL−1) | Concentrate Percentagem (%) | Efficiency (%) | Wp (g g−1) | We (g g−1) | RMS (%) |
|---|---|---|---|---|---|---|---|
| Anthocyanin-rich extract | 3.55 ± 0.14 | - | - | - | - | - | - |
| 1 | 6.29 ± 0.10 | 1.19 ± 0.10 | 49.07 | 80.98 | 0.54 | 0.51 | 2.02 |
| 2 | 9.02± 0.58 | 3.06 ± 0.58 | 51.69 | 66.12 | 0.46 | 0.48 | 1.72 |
| 3 | 9.29 ± 0.42 | 5.43± 0.42 | 85.83 | 41.61 | 0.07 | 0.14 | 5.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuasnei, M.; Benvenutti, L.; Fernando dos Santos, D.; Ferreira, S.R.S.; Pinto, V.Z.; Ferreira Zielinski, A.A. Efficient Anthocyanin Recovery from Black Bean Hulls Using Eutectic Mixtures: A Sustainable Approach for Natural Dye Development. Foods 2024, 13, 1374. https://doi.org/10.3390/foods13091374
Kuasnei M, Benvenutti L, Fernando dos Santos D, Ferreira SRS, Pinto VZ, Ferreira Zielinski AA. Efficient Anthocyanin Recovery from Black Bean Hulls Using Eutectic Mixtures: A Sustainable Approach for Natural Dye Development. Foods. 2024; 13(9):1374. https://doi.org/10.3390/foods13091374
Chicago/Turabian StyleKuasnei, Mayara, Laís Benvenutti, David Fernando dos Santos, Sandra Regina Salvador Ferreira, Vânia Zanella Pinto, and Acácio Antonio Ferreira Zielinski. 2024. "Efficient Anthocyanin Recovery from Black Bean Hulls Using Eutectic Mixtures: A Sustainable Approach for Natural Dye Development" Foods 13, no. 9: 1374. https://doi.org/10.3390/foods13091374