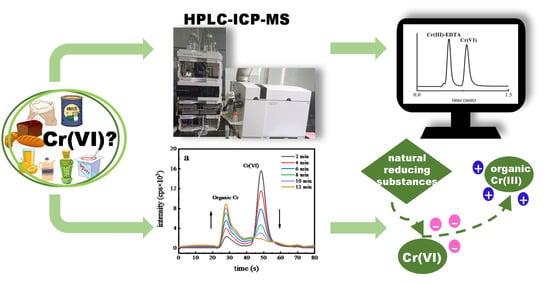
Development of a Fast Method Using Inductively Coupled Plasma Mass Spectrometry Coupled with High-Performance Liquid Chromatography and Exploration of the Reduction Mechanism of Cr(VI) in Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Extraction
2.3. HPLC-ICP-MS Determination
2.4. Method Performance Evaluation
2.5. Stability Study of Cr(VI) in Food Components
3. Results and Discussion
3.1. Optimization of Chromatographic Separation with HPLC-ICP-MS
3.2. Analytical Performance
3.3. Detection of Cr(VI) in Food Samples
3.4. Influence of Food Components and Processing on the Reduction of Cr(VI)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milačič, R.; Ščančar, J. Cr speciation in foodstuffs, biological and environmental samples: Methodological approaches and analytical challenges-A critical review. TrAC Trends Anal. Chem. 2020, 127, 115888. [Google Scholar] [CrossRef]
- Hu, L.; Cai, Y.; Jiang, G. Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environmental samples. Chemosphere 2016, 156, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, N.; Keshava, N.; Chen, J.; Akerman, G.; Kligerman, A.; Rinde, E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: Chromium (VI). Environ. Mol. Mutagen. 2010, 51, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B. Health effects of chromium and its concentrations in cereal foods together with sulfur. Expo. Health 2019, 12, 153–161. [Google Scholar] [CrossRef]
- Mathebula, M.W.; Mandiwana, K.; Panichev, N. Speciation of chromium in bread and breakfast cereals. Food Chem. 2017, 217, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Soares, M.E.; Kozior, M.; Krejpcio, Z.; Ferreira, I.M.; Bastos, M.L. Quantification of total and hexavalent chromium in lager beers: Variability between styles and estimation of daily intake of chromium from beer. J. Agric. Food Chem. 2014, 62, 9195–9200. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; Imran, U.; Ullah, A.; Ullman, J.L.; Weidhaas, J. Health risks associated with accumulation of heavy metals in fish of Keenjhar Lake, Pakistan. Environ. Sci. Pollut. 2020, 27, 24162–24172. [Google Scholar] [CrossRef]
- Novotnik, B.; Zuliani, T.; Ščančar, J.; Milačič, R. Chromate in food samples: An artefact of wrongly applied analytical methodology? J. Anal. At. Spectrom. 2013, 28, 558. [Google Scholar] [CrossRef]
- Vacchina, V.; de la Calle, I.; Seby, F. Cr(VI) speciation in foods by HPLC-ICP-MS: Investigation of Cr(VI)/food interactions by size exclusion and Cr(VI) determination and stability by ion-exchange on-line separations. Anal. Bioanal. Chem. 2015, 407, 3831–3839. [Google Scholar] [CrossRef]
- Chen, Z.F.; Guan, X.; Zhao, Y.S. Morphology analysis of hexavalent chromium reduction to trivalent chromium with syrup under different pH conditions. Appl. Water Sci. 2019, 9, 187. [Google Scholar] [CrossRef]
- Kovacs, R.; Beni, A.; Karosi, R.; Sogor, C.; Posta, J. Investigation of chromium content in foodstuffs and nutrition supplements by GFAAS and determination of changing Cr(III) to Cr(VI) during baking and toasting bread. Food Chem. 2007, 105, 1209–1213. [Google Scholar] [CrossRef]
- Han, T.U.; Kim, J.; Kim, K. Accelerated chromate reduction by tea waste: Comparison of chromate reduction properties between water and ice systems. Environ. Res. 2021, 197, 111059. [Google Scholar] [CrossRef]
- Thor, M.Y.; Harnack, L.; King, D.; Jasthi, B.; Pettit, J. Evaluation of the comprehensiveness and reliability of the chromium composition of foods in the literature. J. Food Compost. Anal. 2011, 24, 1147–1152. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Liu, J.; Han, Y.; Ma, S.; Chen, X. Ratiometric fluorescent detection of chromium(VI) in real samples based on dual emissive carbon dots. Talanta 2018, 185, 249–257. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, M.; Chen, B.; Hu, B. Preparation of functional magnetic porous organic polymer as sorbent for mercury speciation followed by HPLC-ICP-MS analysis. J. Anal. At. Spectrom. 2021, 36, 1568–1575. [Google Scholar] [CrossRef]
- Wen, S.; Zhu, X.; Wu, X.; Qin, X. Directly suspended droplet microextraction coupled with electrothermal atomic absorption spectrometry for the speciation of chromium(iii)/chromium(vi). Anal. Methods 2014, 6, 9777–9782. [Google Scholar] [CrossRef]
- Elamin, M.B.; Chrouda, A.; Ali, S.M.A.; Alhaidari, L.M.; Jabli, M.; Alrouqi, R.M.; Renault, N.J. Electrochemical sensor based on gum Arabic nanoparticles for rapid and in-situ detection of different heavy metals in real samples. Heliyon 2024, 10, e26364. [Google Scholar] [CrossRef]
- Erarpat, S.; Demir, C.; Oner, M.; Bakirdere, S. Chromium speciation by the combination of high-performance liquid chromatography and inductively coupled plasma-optical emission spectrometry. Environ. Monit. Assess. 2022, 194, 690. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, J. Rapid sample preparation with multi-channel focused infrared micro-ashing prior to determination of chromium in gelatin capsules by electrothermal atomic absorption spectrometry. Anal. Methods 2018, 10, 920–925. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, J.J.; Liu, J.H.; Chen, M.L.; Wang, J.H. Determination of chromium based on laser ablation inductively coupled plasma mass spectrometry. Chin. J. Anal. Chem. 2021, 49, 432–439. [Google Scholar] [CrossRef]
- Liu, L.H.; Yang, C.X.; Yan, X.P. Methacrylate-bonded covalent-organic framework monolithic columns for high performance liquid chromatography. J. Chromatogr. A 2017, 1479, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; He, M.; Chen, B.; Xu, C.; Zhang, Q.; Ma, J.; Feng, Y.; Cui, Z. Elemental mass spectrometry in food and environmental chemistry. Mass Spectrom. Food Environ. Chem. 2022, 119, 53–97. [Google Scholar] [CrossRef]
- Xu, F.; Hu, J.; Zhang, J.; Hou, X.; Jiang, X. Nanomaterials in speciation analysis of mercury, arsenic, selenium, and chromium by analytical atomic/molecular spectrometry. Appl. Spectrosc. Rev. 2017, 53, 333–348. [Google Scholar] [CrossRef]
- Hoy, K.S.; Davydiuk, T.; Chen, X.; Lau, C.; Schofield, J.R.M.; Lu, X.; Graydon, J.A.; Mitchell, R.; Reichert, M.; Le, X.C. Arsenic speciation in freshwater fish: Challenges and research needs. Food Qual. Saf. 2023, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mihai, O.; Kawamoto, M.S.; LeBlanc, K.L.; Grinberg, P.; Nogueira, A.R.d.A.; Mester, Z. Determination of chromium picolinate and trace hexavalent chromium in multivitamins and supplements by HPLC-ICP-QQQ-MS. J. Food Compos. Anal. 2020, 87, 103421. [Google Scholar] [CrossRef]
- Drinčić, A.; Ščančar, J.; Zuliani, T.; Nikolić, I.; Milačič, R. Simultaneous speciation of chromate, arsenate, molybdate and vanadate in alkaline samples by HPLC-ICP-MS at different concentration levels of vanadate. J. Anal. At. Spectrom. 2017, 32, 2200–2209. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, S.; Yao, L.; Wang, L.; Qu, H.; Wu, Y.; Chen, Y.; Zheng, L. Single-atom nanozyme enabled fast and highly sensitive colorimetric detection of Cr(VI). J. Hazard. Mater. 2021, 408, 124898. [Google Scholar] [CrossRef]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Watts, M.J. Chromium speciation in foodstuffs: A review. Food Chem. 2018, 250, 105–112. [Google Scholar] [CrossRef]
- Mills, C.T.; Bern, C.R.; Wolf, R.E.; Foster, A.L.; Morrison, J.M.; Benzel, W.M. Modifications to EPA method 3060A to improve extraction of Cr(VI) from chromium ore processing residue-contaminated soils. Environ. Sci. Technol. 2017, 51, 11235–11243. [Google Scholar] [CrossRef] [PubMed]
- Letsoalo, M.R.; Godeto, T.W.; Magadzu, T.; Ambushe, A.A. Quantitative Speciation of Arsenic in Water and Sediment Samples from the Mokolo River in Limpopo Province, South Africa. Anal. Lett. 2018, 51, 2763–2777. [Google Scholar] [CrossRef]
- Lin, Y.A.; Jiang, S.J.; Sahayam, A.C.; Huang, Y.L. Speciation of chromium in edible animal oils after microwave extraction and liquid chromatography inductively coupled plasma mass spectrometry. Microchem. J. 2016, 128, 274–278. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Li, X.L.; Han, C.; Li, J.; Jin, R.; Min, J.Z. Relative quantitation of chiral thiol compounds labeled based on isotope novel mass spectrometry probes: Monitoring of the dynamic changes of chiral thiol compounds in human urine during normal, exercise, and rest recovery states. J. Chromatogr. A 2024, 1719, 464757. [Google Scholar] [CrossRef] [PubMed]
- Pechancova, R.; Gallo, J.; Milde, D.; Pluhacek, T. Ion-exchange HPLC-ICP-MS: A new window to chromium speciation in biological tissues. Talanta 2020, 218, 121150. [Google Scholar] [CrossRef] [PubMed]
- Pechancova, R.; Pluhacek, T.; Gallo, J.; Milde, D. Study of chromium species release from metal implants in blood and joint effusion: Utilization of HPLC-ICP-MS. Talanta 2018, 185, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.; Zuliani, T.; Scancar, J.; Milacic, R. Determination of hexavalent Cr in river sediments by speciated isotope dilution inductively coupled plasma mass spectrometry. Sci. Total Environ. 2018, 637–638, 1286–1294. [Google Scholar] [CrossRef]
- Saraiva, M.; Chekri, R.; Guerin, T.; Sloth, J.J.; Jitaru, P. Chromium speciation analysis in raw and cooked milk and meat samples by species-specific isotope dilution and HPLC-ICP-MS. Food Addit. Contam. A 2021, 38, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Novotnik, B.; Zuliani, T.; Scancar, J.; Milacic, R. Content of trace elements and chromium speciation in Neem powder and tea infusions. J. Trace Elem. Med. Biol. 2015, 31, 98–106. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Reeves, K. Reduction of hexavalent chromium by green tea polyphenols and green tea nano zero-valent iron (GT-nZVI). Bull. Environ. Contam. Toxicol. 2017, 98, 353–358. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhao, Y.S.; Zhang, J.W.; Bai, J. Mechanism and kinetics of hexavalent chromium chemical reduction with sugarcane molasses. Water Air Soil. Pollut. 2015, 226, 363. [Google Scholar] [CrossRef]
- de Souza, F.B.; de Lima Brandão, H.; Hackbarth, F.V.; de Souza, A.A.U.; Boaventura, R.A.R.; de Souza, S.M.A.G.U.; Vilar, V.J.P. Marine macro-alga Sargassum cymosum as electron donor for hexavalent chromium reduction to trivalent state in aqueous solutions. Chem. Eng. J. 2016, 283, 903–910. [Google Scholar] [CrossRef]
- Atapour, M.; Odnevall Wallinder, I.; Hedberg, Y. Stainless steel in simulated milk and whey protein solutions-Influence of grade on corrosion and metal release. Electrochim. Acta 2020, 331, 135428. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Reduction behavior of chromium(VI) with oxalic acid in aqueous solution. Sci. Rep. 2020, 10, 17732. [Google Scholar] [CrossRef]
- Mystrioti, C.; Koursari, S.; Xenidis, A.; Papassiopi, N. Hexavalent chromium reduction by gallic acid. Chemosphere 2021, 273, 129737. [Google Scholar] [CrossRef]






| Parameter | Settings | |
|---|---|---|
| HPLC | Column | WAX 50 mm × 4.6 mm, 5 μm |
| Mobile phase | 70 mM, pH 7, NH4NO3 | |
| Flow rate of the mobile phase | 0.8 mL min−1 | |
| Injection volume | 5 μL | |
| Column temperature | Ambient | |
| ICP-MS | Radio frequency power | 1550 W |
| Carrier gas flow rate | He, 1.0 L min−1 | |
| Compensatory gas flow rate | 1.0 L min−1 | |
| Acquisition mode | Time-resolved data acquisition | |
| Scanning mode | Peak-hopping | |
| Integration mode | Peak area | |
| Monitored isotopes | m/z = 52(Cr) |
| System | Column Type | Analysis Time (min) | LOD (μg kg−1) | References |
|---|---|---|---|---|
| HPLC-ICP-OES | Alltech Allsep (4.6 × 150 mm, 7 µm) | 5.0 | 50.0 | [19] |
| HPLC-ICP-MS | Hamilton PRP-X100 (2.1 × 150 mm, 5 μm) | 11.0 | 30.0 | [35] |
| HPLC-ICP-MS | Agilent ZORBAX Eclipse XDB-C8 (2.1 × 150 mm, 5 µm) | 5.0 | 0.2 | [36] |
| HPLC-ICP-MS | GE Healthcare Mono Q HR 5/5 (5 × 50 mm, 10 μm) | 10.0 | 1.3 | [37] |
| HPLC-ICP-MS | Sepax Proteomix WAX-NP5 (4.6 × 50 mm, 5 μm) | 1.5 | 0.1 | This study |
| Sample | Spiked Level (μg kg−1) | Recovery Rates (%) | ||
|---|---|---|---|---|
| 1: Spiking of Cr(VI) Into the Alkaline Extraction Solution | 2: Spiking of Cr(VI) Into the Food Samples and Mixing for 2 min | 3: Spiking of Cr(VI) Into the Food Samples and Mixing for 3 h | ||
| milk powder | 5 | 104.67 ± 3.60 a | 95.57 ± 6.35 a | 81.88 ± 3.59 b |
| 25 | 100.27 ± 1.43 a | 96.37 ± 3.23 a | 71.46 ± 3.23 b | |
| 50 | 102.04 ± 1.70 a | 100.21 ± 5.11 a | 61.42 ± 2.18 b | |
| rice flour | 5 | 104.80 ± 1.54 a | 103.83 ± 1.41 a | 99.89 ± 2.05 a |
| 25 | 100.91 ± 3.61 a | 99.47 ± 0.24 a | 102.38 ± 0.76 a | |
| 50 | 95.89 ± 1.80 ab | 99.33 ± 5.42 a | 91.96 ± 1.79 b | |
| whole wheat bread | 5 | 105.36 ± 1.41 a | 103.68 ± 4.90 a | 85.82 ±1.41 b |
| 25 | 103.97 ± 0.65 a | 104.76 ± 0.67 a | 98.76 ± 0.52 b | |
| 50 | 101.89 ± 5.78 a | 100.22 ± 6.50 a | 93.08 ± 0.70 b | |
| yoghurt | 5 | 111.85 ± 2.18 a | 84.55 ± 4.71 b | 76.13 ± 5.01 c |
| 25 | 107.17 ± 1.35 a | 92.05 ± 3.32 b | 78.17 ± 3.72 c | |
| 50 | 111.03 ± 3.01 a | 90.11 ± 2.43 b | 86.43 ± 1.42 c | |
| white vinegar | 5 | 93.76 ± 2.18 a | 73.96 ± 2.13 b | 40.44 ± 0.37 c |
| 25 | 104.30 ±1.80 a | 98.28 ± 1.26 b | 76.24 ± 0.64 c | |
| 50 | 103.48 ± 2.44 a | 93.58 ± 1.86 b | 76.67 ± 2.52 c | |
| orange juice | 5 | 91.70 ± 1.48 | 33.91 ± 1.12 | not detected |
| 25 | 105.62 ± 1.29 | 90.41 ± 2.14 | not detected | |
| 50 | 106.02 ± 2.77 | 86.68 ± 2.59 | not detected | |
| green tea | 5 | 98.87 ± 1.09 | 79.26 ± 1.93 | not detected |
| 25 | 106.92 ± 0.98 | 80.03 ± 0.79 | not detected | |
| 50 | 109.69 ± 1.72 | 86.88 ± 0.84 | not detected | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Tan, H.; Cheng, C.; Li, P.; Sun, X.; Zhou, Y.; Fang, Y. Development of a Fast Method Using Inductively Coupled Plasma Mass Spectrometry Coupled with High-Performance Liquid Chromatography and Exploration of the Reduction Mechanism of Cr(VI) in Foods. Toxics 2024, 12, 325. https://doi.org/10.3390/toxics12050325
Song G, Tan H, Cheng C, Li P, Sun X, Zhou Y, Fang Y. Development of a Fast Method Using Inductively Coupled Plasma Mass Spectrometry Coupled with High-Performance Liquid Chromatography and Exploration of the Reduction Mechanism of Cr(VI) in Foods. Toxics. 2024; 12(5):325. https://doi.org/10.3390/toxics12050325
Chicago/Turabian StyleSong, Ge, Honglian Tan, Chuhan Cheng, Peng Li, Xinyang Sun, Yuling Zhou, and Yong Fang. 2024. "Development of a Fast Method Using Inductively Coupled Plasma Mass Spectrometry Coupled with High-Performance Liquid Chromatography and Exploration of the Reduction Mechanism of Cr(VI) in Foods" Toxics 12, no. 5: 325. https://doi.org/10.3390/toxics12050325