Eco-Friendly Sol–Gel Coatings with Organic Corrosion Inhibitors for Lightweight AZ61 Alloy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coatings Characterization
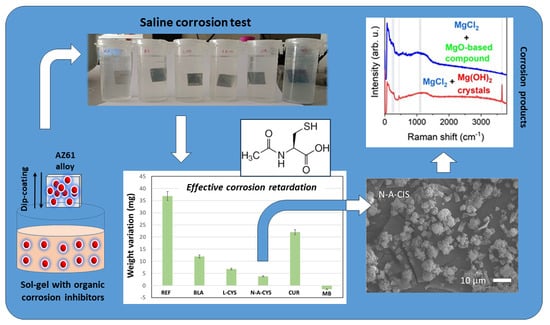
2.2. Weathering Test
2.3. Optical Microscopy Characterization
2.4. Weight Variation
2.5. SEM Characterization
2.6. Confocal Raman Microscopy Characterization
3. Conclusions
4. Materials and Methods
4.1. AZ61 Alloy
4.2. Synthesis of the Sol–Gel Coatings for Inhibitory Performances
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, D.; Rubio, E.M.; Sáenz-Nuño, M.A.; Lorente-Pedreille, R.M. Comparison of sustainable cooling systems used in the drilling repair of Mg-Al and Mg-Ti multi-material parts in the aeronautical industry. Tribol. Int. 2022, 175, 107804. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Santos, L.S.; Vitry, V.; Paint, Y.; Olivier, M. Improvement of the corrosion performance of AA2024 alloy by a duplex PEO/clay modified sol-gel nanocomposite coating. Surf. Coat. Technol. 2022, 434, 128168. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.-L.; Atrens, A. Influence of Homogenization Annealing of AZ91 on MechanicalProperties and Corrosion Behavior. Adv. Eng. Mater. 2008, 10, 93–103. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, R.K.; Sharma, V.; Minhas, N.; Thakur, A. Review: Corrosion behavior of friction stir welded magnesium alloys. Mater. Today Proc. 2023, 80, 272–277. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Llorente, I. Corrosion product layers on magnesium alloys AZ31 and AZ61: Surface chemistry and protective ability. Appl. Surf. Sci. 2015, 347, 736–746. [Google Scholar] [CrossRef]
- Kumar, P.P.; Bharat, A.R.; Sai, B.S.; Sarath, R.J.P.; Akhil, P.; Reddy, G.K.; Kondaiah, V.V.; Sunil, B.R. Role of microstructure and secondary phase on corrosion behavior of heat treated AZ series magnesium alloys. Mater. Today Proc. 2019, 18, 175–181. [Google Scholar] [CrossRef]
- Dislich, H.; Hinz, P. History and principles of the sol-gel process, and some new multicomponent oxide coatings. J. Non-Cryst. Solids 1982, 48, 11–16. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Senani, S.; Campazzi, E.; Villatte, M.; Druez, C. Potentiality of UV-cured Hybrid sol-gel coatings for aeronautical metallic substrate protection. Surf. Coat. Technol. 2013, 227, 32–37. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Ma, H.; Das, S.; Gu, Y.; Zhang, L. Probing local corrosion performance of sol-gel/MAO composite coating on Mg alloy. Surf. Coat. Technol. 2018, 347, 286–296. [Google Scholar] [CrossRef]
- Murillo-Gutiérrez, N.V.; Ansart, F.; Bonino, J.-P.; Menu, M.-J.; Gressier, M. Protection against corrosion of magnesium alloys with both conversion layer and sol-gel coating. Surf. Coat. Technol. 2013, 232, 606–615. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galván, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to Organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, L.; López-Sánchez, J.; Serrano, A.; Rodríguez de la Fuente, O.; Galván, J.C.; Carmona, N. Hybrid sol-gel coatings doped with non-toxic corrosion inhibitors for corrosion protection on AZ61 magnesium alloy. Gels 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, F.; Zhang, D. A study on corrosion inhibitor for magnesium alloy. Mater. Sci. Forum 2009, 610–613, 920–926. [Google Scholar] [CrossRef]
- Umoren, S.A.; Abdullahi, M.T.; Solomon, M.M. An overview on the use of corrosion inhibitors for the corrosion control of Mg and its alloys in diverse media. J. Mater. Res. Technol. 2022, 20, 2060–2093. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Jafari, R.; Kazempour, A. Effect of amino acids and montmorillonite nanoparticles on improving the corrosion protection characteristics of Hybrid sol-gel coating Applied on AZ91 Mg alloy. Mater. Chem. Phys. 2019, 225, 298–308. [Google Scholar] [CrossRef]
- BinSabt, M.; Abditon, M.; Galal, A. Highly efficient nanocomposite-containing coatings for the protection of Mg alloy against corrosion in chloride containing electrolytes. Appl. Phys. A 2022, 128, 151. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Zeng, Y.; Chen, C.; Guo, H.; Lei, B.; Zhang, P.; Feng, Z.; Meng, G. A novel sol-gel coating via catechol/lysine polymerization for long-lasting corrosion protection of Mg alloy AZ31. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130361. [Google Scholar] [CrossRef]
- Passos, M.L.C.; Saraiva, M.L.M.F.S. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Montero, E.F.; García, M.A.; Villegas Broncano, M.A.; Llopis, J. Estudio de las propiedades ópticas de recubrimientos Sol-Gel dopados con fluoresceína en función de la concentración y del pH. Boletín Soc. Española Cerámica Vidr. 2004, 43, 8–11. Available online: http://boletines.secv.es/upload/2007013193009.43[1]8-11.pdf (accessed on 22 September 2022). [CrossRef]
- ISO Standard 11130; Corrosion of Metals and Alloys—Alternate Immersion Test in Salt Solution. 2017. Available online: http://www.iso.org (accessed on 17 April 2023).
- Solmaz, R. Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1,3-dienylideneamino)-1,3,4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid, Corrosion. Science 2010, 52, 3321–3333. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, S.G.; Wang, F. Understanding corrosion inhibition mechanisms—Experimental and theoretical approach. RSC Adv. 2011, 1, 866–873. [Google Scholar] [CrossRef]
- Udabe, E.; Somers, A.; Forsyth, M.; Mecerreyes, D. Design of polymeric corrosion inhibitors based on ionic coumarate groups. Appl. Polym. Mater. 2021, 3, 1739–1746. [Google Scholar] [CrossRef]
- ASTM Standard G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals (Reapproved 1990). Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2004; p. 302.
- Aparicio, M.; Mosa, J.; Rodríguez, G.; Guzman, J.; Picard, Q.; Klein, L.C.; Jitianu, A. Consolidated Melting Gel Coatings on AZ31 Magnesium Alloy with Excellent Corrosion Resistance in NaCl Solutions: An Interface Study. ACS Appl. Mater. Interfaces 2019, 11, 3493–3505. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, J.; Serrano, A.; Del Campo, A.; Abuín, M.; Rodríguez de la Fuente, O.; Carmona, N. Sol–Gel Synthesis and Micro-Raman Characterization of ε-Fe2O3 Micro- and Nanoparticles. Chem. Mater. 2016, 28, 511–518. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Serrano, A.; del Campo, A.; Abuín, M.; Salas-Colera, E.; Muñoz-Noval, A.; Castro, G.R.; de la Figuera, J.; Marco, J.F.; Marín, P.; et al. Self-assembly of iron oxide precursor micelles driven by magnetic stirring time in sol–gel coatings. RSC Adv. 2019, 9, 17571. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, J.; Serrano, A.; del Campo, A.; Muñoz-Noval, Á.; Salas-Colera, E.; Cabero, M.; Varela, M.; Abuín, M.; Castro, G.R.; Rubio-Zuazo, J.; et al. A combined micro-Raman, X-ray absorption and magnetic study to follow the glycerol-assisted growth of epsilon-iron oxide sol-gel coatings. J. Alloys Compd. 2022, 892, 162061. [Google Scholar] [CrossRef]
- Dekermenjian, M.; Ruediger, A.P.; Merlen, A. Raman spectroscopy investigation of magnesium oxide nanoparticles. RSC Adv. 2023, 13, 26683. [Google Scholar] [CrossRef]
- Ishikawa, K.; Fujima, N.; Komura, H. First-order Raman scattering in MgO microcrystals. J. Appl. Phys. 1985, 57, 973. [Google Scholar] [CrossRef]
- Sangster, M.J.L.; Peckham, G.; Saunderson, D.H. Lattice dynamics of magnesium oxide. J. Phys. C Solid State Phys. 1970, 3, 1026. [Google Scholar] [CrossRef]
- Dawson, P.; Hadfield, C.D.; Wilkinson, G.R. The polarized infra-red and Raman spectra of Mg(OH)2 and Ca(OH)2. J. Phys. Chem. Solids 1973, 34, 1217–1225. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Vannoy, C.H.; Leblanc, R.M.; Wang, D.Z. Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram Int. 2009, 35, 3355–3364. [Google Scholar] [CrossRef]
- Sano, H.; Miyaoka, H.; Kuze, T.; Mori, H.; Mizutani, G.; Otsuka, N.; Terano, M. Raman Spectra of Magnesium Chloride Thin Films. Surf. Sci. 2002, 502–503, 70–74. [Google Scholar] [CrossRef]











| Sample Name | Sol–Gel Coating | Corrosion Inhibitor Added | Sol’s Color | Sol’s pH | Corrosion Test Performed |
|---|---|---|---|---|---|
| A | NO | - | - | - | NO |
| REF | NO | - | - | - | YES |
| BLA | YES | - | Pink | 2 | YES |
| L-CYS | YES | L-cysteine | Pink | 3–4 | YES |
| N-A-CYS | YES | N-acetyl-cysteine | Red | 2 | YES |
| CUR | YES | Curcumin | Brown | 2 | YES |
| MB | YES | Methylene Blue | Blue | 3 | YES |
| Sample | BLA | L-CYS | N-A-CYS | CUR | MB |
|---|---|---|---|---|---|
| Thickness (nm) | 195 ± 30 | 291 ± 40 | 281 ± 57 | 164 ± 30 | 268 ± 42 |
| Sample | C (wt.%) | O (wt.%) | Mg (wt.%) | Al (wt.%) | Si (wt.%) | Cl (wt.%) |
|---|---|---|---|---|---|---|
| A-background | - | - | 92.5 ± 1.7 | 7.5 ± 1.7 | - | - |
| REF-background | - | 57.3 ± 2.4 | 42.7 ± 2.4 | - | - | - |
| REF-white goop | - | 29.7 ± 2.7 | 66.7 ± 2.7 | 3.7 ± 1.2 | - | - |
| REF-crystal | - | 57.9 ± 2.4 | 42.1 ± 2.4 | - | - | - |
| BLA-background | - | 57.8 ± 2.5 | 39.5 ± 2.4 | - | - | 2.7 ± 0.8 |
| BLA-triangle | - | 57.4 ± 2.2 | 42.6 ± 2.2 | - | - | - |
| L-CYS-background | - | 23.0 ± 2.6 | 77.0 ± 2.6 | - | - | - |
| L-CYS-smooth area | - | 14.7 ± 2.5 | 78.3 ± 2.7 | 5.8 ± 1.4 | 1.2 ± 1.0 | - |
| L-CYS-stick | - | 51.7 ± 2.0 | 46.1 ± 2.0 | 2.3 ± 0.7 | - | - |
| MB-background | - | 38.1 ± 1.3 | 50.1 ± 1.2 | 2.2 ± 0.5 | 9.6 ± 0.7 | - |
| CUR-smooth area | 19.2 ± 2.4 | 17.7 ± 1.1 | 60.3 ± 2.0 | 2.8 ± 0.3 | - | - |
| CUR-crust area | - | 57.0 ± 1.1 | 43.0 ± 1.1 | - | - | - |
| N-A-CYS-background | 9.0 ± 2.1 | 43.1 ± 1.4 | 43.1 ± 1.3 | 1.1 ± 0.3 | 2.7 ± 0.3 | 1.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Martínez, J.; López-Sánchez, J.; García-Galván, F.; Serrano, A.; Barranco, V.; Galván, J.C.; Rodríguez de la Fuente, Ó.; Carmona, N. Eco-Friendly Sol–Gel Coatings with Organic Corrosion Inhibitors for Lightweight AZ61 Alloy. Gels 2024, 10, 168. https://doi.org/10.3390/gels10030168
Domínguez-Martínez J, López-Sánchez J, García-Galván F, Serrano A, Barranco V, Galván JC, Rodríguez de la Fuente Ó, Carmona N. Eco-Friendly Sol–Gel Coatings with Organic Corrosion Inhibitors for Lightweight AZ61 Alloy. Gels. 2024; 10(3):168. https://doi.org/10.3390/gels10030168
Chicago/Turabian StyleDomínguez-Martínez, Jorge, Jesús López-Sánchez, Federico García-Galván, Aída Serrano, Violeta Barranco, Juan Carlos Galván, Óscar Rodríguez de la Fuente, and Noemí Carmona. 2024. "Eco-Friendly Sol–Gel Coatings with Organic Corrosion Inhibitors for Lightweight AZ61 Alloy" Gels 10, no. 3: 168. https://doi.org/10.3390/gels10030168