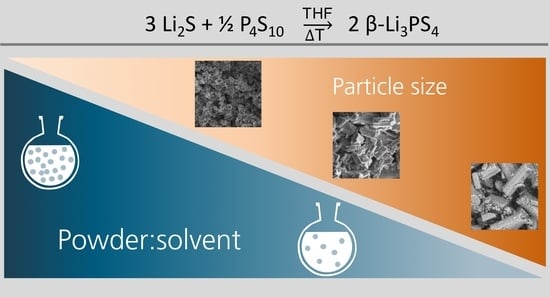
Influence of Solid Fraction on Particle Size during Wet-Chemical Synthesis of β-Li3PS4 in Tetrahydrofuran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of β-Li3PS4
2.2. Characterization
2.2.1. XRD
2.2.2. SEM
2.2.3. Electrochemical Impedance Measurements
3. Results
3.1. Synthesis
3.2. XRD
3.3. SEM
3.4. Impedance Spectroscopy
4. Discussion on Solid Deposition and Particle Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.V.; Hu, Y.-S.; Li, H.; Janek, J.; Nan, C.; Nazar, L.; Maier, J.; Armand, M.; et al. New Horizons for Inorganic Solid State Ion Conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 30. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Stöffler, H.; Zinkevich, T.; Yavuz, M.; Hansen, A.-L.; Knapp, M.; Bednarčík, J.; Randau, S.; Richter, F.H.; Janek, J.; Ehrenberg, H.; et al. Amorphous versus Crystalline Li3PS4: Local Structural Changes during Synthesis and Li Ion Mobility. J. Phys. Chem. C 2019, 123, 10280–10290. [Google Scholar] [CrossRef]
- Ghidiu, M.; Ruhl, J.; Culver, S.P.; Zeier, W.G. Solution-based synthesis of lithium thiophosphate superionic conductors for solid-state batteries: A chemistry perspective. J. Mater. Chem. A 2019, 7, 17735–17753. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Yu, Z.; Bowden, M.; Miller, Q.R.; Chen, P.; Schaef, H.T.; Mueller, K.T.; Lu, D.; Xiao, J.; et al. Wet-chemical synthesis of Li7P3S11 with tailored particle size for solid state electrolytes. Chem. Eng. J. 2022, 429, 132334. [Google Scholar] [CrossRef]
- Shi, T.; Tu, Q.; Tian, Y.; Xiao, Y.; Miara, L.J.; Kononova, O.; Ceder, G. High Active Material Loading in All-Solid-State Battery Electrode via Particle Size Optimization. Adv. Energy Mater. 2020, 10, 1902881. [Google Scholar] [CrossRef]
- Mertens, A.; Yu, S.; Schön, N.; Gunduz, D.C.; Tempel, H.; Schierholz, R.; Hausen, F.; Kungl, H.; Granwehr, J.; Eichel, R.-A. Superionic bulk conductivity in Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Solid State Ion. 2017, 309, 180–186. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takahashi, M.; Ohara, K.; Phuc, N.H.H.; Yang, S.; Watanabe, T.; Uchiyama, T.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; et al. Synthesis of Sulfide Solid Electrolytes through the Liquid Phase: Optimization of the Preparation Conditions. ACS Omega 2020, 5, 26287–26294. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yang, S.; Takahashi, M.; Ohara, K.; Uchiyama, T.; Watanabe, T.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; Muto, H.; et al. High Ionic Conductivity of Liquid-Phase-Synthesized Li3PS4 Solid Electrolyte, Comparable to That Obtained via Ball Milling. ACS Appl. Energy Mater. 2021, 4, 2275–2281. [Google Scholar] [CrossRef]
- Ohara, K.; Masuda, N.; Yamaguchi, H.; Yao, A.; Tominaka, S.; Yamada, H.; Hiroi, S.; Takahashi, M.; Yamamoto, K.; Wakihara, T.; et al. Observation of Liquid Phase Synthesis of Sulfide Solid Electrolytes Using Time-Resolved Pair Distribution Function Analysis. Phys. Status Solidi B 2020, 257, 2000106. [Google Scholar] [CrossRef]
- Ito, A.; Kimura, T.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Liquid-phase synthesis of Li3PS4 solid electrolyte using ethylenediamine. J. Sol.-Gel. Sci. Technol. 2021, 414, 359. [Google Scholar] [CrossRef]
- Calpa, M.; Nakajima, H.; Mori, S.; Goto, Y.; Mizuguchi, Y.; Moriyoshi, C.; Kuroiwa, Y.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Formation Mechanism of β-Li3PS4 through Decomposition of Complexes. Inorg. Chem. 2021, 60, 6964–6970. [Google Scholar] [CrossRef] [PubMed]
- Delnick, F.M.; Yang, G.; Self, E.C.; Meyer, H.M.; Nanda, J. Investigation of Complex Intermediates in Solvent-Mediated Synthesis of Thiophosphate Solid-State Electrolytes. J. Phys. Chem. C 2020, 124, 27396–27402. [Google Scholar] [CrossRef]
- Gries, A.; Langer, F.; Schwenzel, J.; Busse, M. Determination of Reaction Enthalpies of Synthesizing β-Li3PS4 in Tetrahydrofuran. ACS Omega 2023, 8, 14034–14040. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Evans, E.H.; Smith, D.K. The reference intensity ratio, I/IC, for computer simulated powder patterns. J. Appl. Crystallogr. 1976, 9, 169–174. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. In Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen; Vandenhoeck & Ruprecht: Göttingen, Germany, 1918; pp. 98–100. [Google Scholar]
- Stöffler, H.; Zinkevich, T.; Yavuz, M.; Senyshyn, A.; Kulisch, J.; Hartmann, P.; Adermann, T.; Randau, S.; Richter, F.H.; Janek, J.; et al. Li+-Ion Dynamics in β-Li3PS4 Observed by NMR: Local Hopping and Long-Range Transport. J. Phys. Chem. C 2018, 122, 15954–15965. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-D.; Yue, X.; Xing, X.; Petrova, V.; Gonzalez, M.; Liu, H.; Liu, P. Designing solution chemistries for the low-temperature synthesis of sulfide-based solid electrolytes. J. Mater. Chem. A 2018, 6, 7370–7374. [Google Scholar] [CrossRef]
- Suto, K.; Bonnick, P.; Nagai, E.; Niitani, K.; Arthur, T.S.; Muldoon, J. Microwave-aided synthesis of lithium thiophosphate solid electrolyte. J. Mater. Chem. A 2018, 6, 21261–21265. [Google Scholar] [CrossRef]
- Cronau, M.; Szabo, M.; König, C.; Wassermann, T.B.; Roling, B. How to Measure a Reliable Ionic Conductivity? The Stack Pressure Dilemma of Microcrystalline Sulfide-Based Solid Electrolytes. ACS Energy Lett. 2021, 6, 3072–3077. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.S.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Wang, Y.; Feng, Z.; Wang, Y.; Lü, X.; Zhu, J.; Ren, Y.; Liang, C. In-situ investigation of pressure effect on structural evolution and conductivity of Na3SbS4 superionic conductor. J. Power Sources 2018, 401, 111–116. [Google Scholar] [CrossRef]
- Schneider, C.; Schmidt, C.P.; Neumann, A.; Clausnitzer, M.; Sadowski, M.; Harm, S.; Meier, C.; Danner, T.; Albe, K.; Latz, A.; et al. Effect of Particle Size and Pressure on the Transport Properties of the Fast Ion Conductor t-Li7SiPS8. Adv. Energy Mater. 2023, 13, 2203873. [Google Scholar] [CrossRef]
- Ohno, S.; Bernges, T.; Buchheim, J.; Duchardt, M.; Hatz, A.-K.; Kraft, M.A.; Kwak, H.; Santhosha, A.L.; Liu, Z.; Minafra, N.; et al. How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Lett. 2020, 5, 910–915. [Google Scholar] [CrossRef]
- Milan, E.; Pasta, M. The role of grain boundaries in solid-state Li-metal batteries. Mater. Futures 2023, 2, 13501. [Google Scholar] [CrossRef]
- Arai, Y. Chemistry of Powder Production; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- von Weimarn, P.P. The Precipitation Laws. Chem. Rev. 1925, 2, 217–242. [Google Scholar] [CrossRef]





| Sample-200 | Sample-100 | Sample-50 | |
|---|---|---|---|
| Concentration in mg/mL | 200 | 100 | 50 |
| Temperature increase in K | 40 | 21 | 9.6 |
| Residual Li2S in % | 4.7 | 5.7 | 4.1 |
| Crystallite size in nm | 76 ± 9 | 72 ± 7 | 52 ± 5 |
| Largest particles in µm | 9.3 ± 0.7 | 33 ± 4 | 64 ± 5 |
| Ionic conductivity × 10−4 S/cm | 0.63 ± 0.01 | 0.98 ± 0.03 | 0.78 ± 0.05 |
| Activation energy in eV | 0.388 ± 0.002 | 0.379 ± 0.002 | 0.383 ± 0.004 |
| Pellet density in % | 78 ± 1 | 77.4 ± 0.8 | 79.3 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gries, A.; Langer, F.; Schwenzel, J.; Busse, M. Influence of Solid Fraction on Particle Size during Wet-Chemical Synthesis of β-Li3PS4 in Tetrahydrofuran. Batteries 2024, 10, 132. https://doi.org/10.3390/batteries10040132
Gries A, Langer F, Schwenzel J, Busse M. Influence of Solid Fraction on Particle Size during Wet-Chemical Synthesis of β-Li3PS4 in Tetrahydrofuran. Batteries. 2024; 10(4):132. https://doi.org/10.3390/batteries10040132
Chicago/Turabian StyleGries, Aurelia, Frederieke Langer, Julian Schwenzel, and Matthias Busse. 2024. "Influence of Solid Fraction on Particle Size during Wet-Chemical Synthesis of β-Li3PS4 in Tetrahydrofuran" Batteries 10, no. 4: 132. https://doi.org/10.3390/batteries10040132