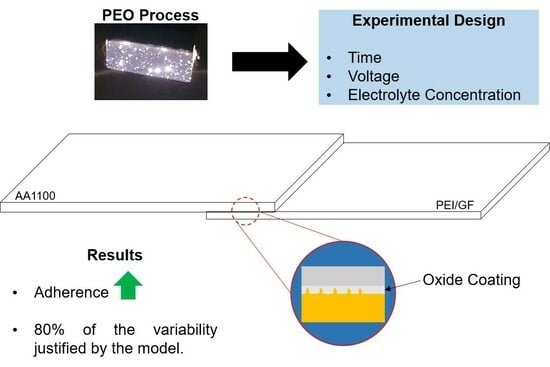
Experimental Design of the Adhesion between a PEI/Glass Fiber Composite and the AA1100 Aluminum Alloy with Oxide Coating Produced via Plasma Electrolytic Oxidation (PEO)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermoplastic Composite Laminates
2.2. Aluminum Alloy 1100
2.3. PEO Surface Treatment Process
2.4. Welding Process
2.5. LSS Test
2.6. Characterizations
2.7. Statistical Analysis
3. Results
3.1. Experimental Design (23)
| Standard | Coded Variables | Real Variables | LSS (MPa) | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Time (s) | Voltage (V) | Concentration (g/L) | ||
| 00 | N/A | N/A | N/A | N/A | N/A | N/A | 5.2 ± 2.2 |
| 01 | −1 | −1 | −1 | 300 | 200 | 3.0 | 8.0 ± 1.2 |
| 02 | +1 | −1 | −1 | 900 | 200 | 3.0 | 7.3 ± 1.0 |
| 03 | −1 | +1 | −1 | 300 | 350 | 3.0 | 10.5 ± 2.3 |
| 04 | +1 | +1 | −1 | 900 | 350 | 3.0 | 9.2 ± 0.9 |
| 05 | −1 | −1 | +1 | 300 | 200 | 10.0 | 7.9 ± 2.4 |
| 06 | +1 | −1 | +1 | 900 | 200 | 10.0 | 10.7 ± 1.1 |
| 07 | −1 | +1 | +1 | 300 | 350 | 10.0 | 9.3 ± 1.1 |
| 08 | +1 | +1 | +1 | 900 | 350 | 10.0 | 8.8 ± 2.4 |
| 09 | −α | 0 | 0 | 95 | 275 | 6.5 | 6.6 ± 1.6 |
| 10 | +α | 0 | 0 | 1105 | 275 | 6.5 | 9.2 ± 1.7 |
| 11 | 0 | −α | 0 | 600 | 150 | 6.5 | 9.1 ± 1.3 |
| 12 | 0 | +α | 0 | 600 | 400 | 6.5 | 7.5 ± 3.9 |
| 13 | 0 | 0 | −α | 600 | 275 | 0.6 | 10.0 ± 1.7 |
| 14 | 0 | 0 | +α | 600 | 275 | 12.0 | 9.7 ± 1.7 |
| 15 | 0 | 0 | 0 | 600 | 275 | 6.5 | 8.7 ± 2.1 |
| 16 | 0 | 0 | 0 | 600 | 275 | 6.5 | 8.2 ± 3.0 |
| 17 | 0 | 0 | 0 | 600 | 275 | 6.5 | 5.4 ± 1.5 |
3.2. Morphological and Chemical Analyses of the More Appropriate Test Pattern
4. Conclusions
- Adhesion between polyetherimide reinforced with glass fiber and 1100 aluminum was significantly improved. Single lap shear tests indeed showed a doubling of shear strength: ~5 to 10 ± 1 MPa. This value is higher than that shown for some methods of joining dissimilar materials.
- Through the analysis of variance, it was found that the statistical model addressed in this study covers approximately 79.5% of all variability in the process of joining materials. This value is attributed to the high percentage of the “error pure” variable, which in this case was 20.5%.
- When evaluating the surface morphology of the plasma-anodized alloy, a coral-like microstructure was observed with pores distributed across the entire surface. However, there was no control over the density of these pores, with a diameter of 0.22 µm, as well as micro-cracks due to the rapid cooling of the liquid oxide coating. Additionally, spherical protrusions were present, and these characteristics have been previously mentioned in the literature.
- The chemical composition on the surface of the oxide coating was essentially composed of elements from the treated alloy. The presence of elements from the electrolyte used is not very evident.
- In line with the analysis of the composition of identified elements, FT-IR investigation revealed the presence of Al-O/Al-O-Si functional groups. After the welding process and with visual evidence of polymer on the surface of the treated aluminum, there were flexural and stretching vibrations of C-N, as well as other functional groups below 1000 cm−1, indicating the possible formation of compounds, such as polymer + oxide.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megahed, M.; El-Baky, M.A.A.; Alsaeedy, A.M.; Alshorbagy, A.E. Improvement of Impact and Water Barrier Properties of GLARE by Incorporation of Different Types of Nanoparticles. Fibers Polym. 2020, 21, 840–848. [Google Scholar] [CrossRef]
- Almeida, R.S.; Damato, C.A.; Botelho, E.C.; Pardini, L.C.; Rezende, M.C. Effect of surface treatment on fatigue behavior of metal/carbon fiber laminates. J. Mater. Sci. 2008, 43, 3173–3179. [Google Scholar] [CrossRef]
- Thomas, L.C.; Kumar, V.; Gangwar, A.; Pisupati, M.; Gupta, C.; Panda, S.K. Computational Modelling and Experimental Techniques for Fibre Metal Laminate Structural Analysis: A Comprehensive Review. Arch Comput. Methods Eng. 2024, 31, 351–369. [Google Scholar] [CrossRef]
- Shore, D.; Wilson, J.C.A.-B.; Matthews, A.; Yerokhin, A. Adhesive bond strength of PEO coated AA6060-T6. Surf. Coat. Technol. 2021, 428, 127898. [Google Scholar] [CrossRef]
- Embraer: News-Embraer–“The Shape of Things to Come, New Sustainable Aircraft Concepts Revealed”. 2022. Available online: https://newsroom.aviator.aero/embraer-the-shape-of-things-to-come-new-sustainable-aircraft-concepts-revealed/ (accessed on 2 December 2023).
- Khalid, M.Y.; Umer, R.; Khan, K.A. Review of recent trends and developments in aluminium 7075 alloy and its metal matrix composites (MMCs) for aircraft applications. Results Eng. 2023, 20, 101372. [Google Scholar] [CrossRef]
- Lucas, R.R. Estudo da Oxidação Eletrolítica a Plasma na liga AA2024-T3 para Soldagem com Compósito PEI/Fibra de vidro. Available online: http://hdl.handle.net/11449/234558 (accessed on 15 June 2023).
- Jadhav, P.; Bongale, A.; Kumar, S. A review of process characteristics of plasma electrolytic oxidation of aluminium alloy. J. Phys. Conf. Ser. 2021, 1, 1854. [Google Scholar] [CrossRef]
- Lucas, R.R.; Mota, R.P.; Abrahão, A.B.R.M.; Botelho, E.C.; Sales-Contini, R.C.M. Characterization of oxide coating grown by plasma electrolytic oxidation (PEO) at different times on aluminum alloy AA2024-T3. MRS Commun. 2022, 12, 266–271. [Google Scholar] [CrossRef]
- Karbasi, M.; Nikoomanzari, E.; Hosseini, R.; Bahramian, H.; Chaharmahali, R.; Giannakis, S.; Kaseem, M.; Fattah-Alhosseini, A. A review on plasma electrolytic oxidation coatings for organic pollutant degradation: How to prepare them and what to expect of them? J. Environ. Chem. Eng. 2023, 11, 110027. [Google Scholar] [CrossRef]
- Christudasjustus, J.; Vukkum, V.; Gupta, R. Evolution of surface film in AA2024-T3 after a long-term immersion in NaCl solution. Corros. Sci. 2023, 215, 111056. [Google Scholar] [CrossRef]
- Kaseem, M.; Dikici, B. Optimization of Surface Properties of Plasma Electrolytic Oxidation Coating by Organic Additives: A Review. Coatings 2023, 11, 374. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-Alhosseini, A.; Karbasi, M.; Giannakis, S.; Bahramian, H.; Oulego, P. A systematic study on modulation of plasma electrolytic oxidation parameters for optimizing photocatalytic coatings on titanium substrates. J. Alloys Compd. 2023, 963, 171234. [Google Scholar] [CrossRef]
- Shahri, Z.; Allahkaram, S.; Soltani, R.; Jafari, H. Optimization of plasma electrolyte oxidation process parameters for corrosion resistance of Mg alloy. J. Magnes. Alloys 2020, 8, 431–440. [Google Scholar] [CrossRef]
- “Taguchi Optimization of Electrolytic Plasma Hardening Process Parameters on Ti-6Al-4V Alloy: Microstructure and Mechanical Properties-Ayday-2023-Advanced Engineering Materials-Wiley Online Library”. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adem.202300896 (accessed on 15 January 2024).
- Vargas, C.A.; Zuleta, A.A.; Botero, C.A.; Baena, L.M.; Castaño, J.G.; Gómez, M.A.; Tamayo, J.A. Morphological analysis of plasma electrolytic oxidation coatings formed on Ti6Al4V alloys manufactured by electron beam powder bed fusion. Heliyon 2023, 9, e19289. [Google Scholar] [CrossRef]
- Oliveira, V.S.; Society, A.W.; Lucas, R.R.; Carvalho, T.P.; Marques, L.F.; Reis, J.F.; Abrahão, A.B.R.M.; Botelho, E.C. Development of the Oxyacetylene Welding Process for PEI/Glass Fiber Laminates. Weld. J. 2021, 100, 142–149. [Google Scholar] [CrossRef]
- Rajak, D.K.; Wagh, P.H.; Linul, E. Manufacturing Technologies of Carbon/Glass Fiber-Reinforced Polymer Composites and Their Properties: A Review. Polymers 2021, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, C.; Xiao, C.; Cheng, J.; Ren, R.; Xiong, X. Heat distribution simulation and effects of ultrasonic welding amplitude on carbon fiber/polyetherimide composite joint properties. Mat. Lett. 2023, 340, 134148. [Google Scholar] [CrossRef]
- Salazar, J.M.G.; Soria, A.; Barrena, M.I. Welding of AA6061-(Al2O3)p composite: Effect of weld process variables and post-welding heat treatment on microstructure and mechanical properties. Sci. Tech. Weld. Join. 2013, 10, 339–343. [Google Scholar] [CrossRef]
- Abibe, A.; Sônego, M.; dos Santos, J.; Canto, L.; Amancio-Filho, S. On the feasibility of a friction-based staking joining method for polymer–metal hybrid structures. Mater. Des. 2016, 92, 632–642. [Google Scholar] [CrossRef]
- Aliasghari, S.; Ghorbani, M.; Skeldon, P.; Karami, H.; Movahedi, M. Effect of plasma electrolytic oxidation on joining of AA 5052 aluminium alloy to polypropylene using friction stir spot welding. Surf. Coat. Technol. 2017, 313, 274–281. [Google Scholar] [CrossRef]
- Schäfer, H.; Blaga, L.; Stöver, E.; Klusemann, B. Refill friction stir spot welding of thermoplastic composites: Case study on Carbon-fiber-reinforced polyphenylene sulfide. Thin-Walled Struct. 2023, 191, 111037. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Kroes, A.D.A.; Finley, J.R. Demystifying omega squared: Practical guidance for effect size in common analysis of variance designs. Psychol. Methods 2023. [Google Scholar] [CrossRef] [PubMed]
- Zehra, T.; Patil, S.A.; Shrestha, N.K.; Fattah-Alhosseini, A.; Kaseem, M. Anionic assisted incorporation of WO3 nanoparticles for enhanced electrochemical properties of AZ31 Mg alloy coated via plasma electrolytic oxidation. J. Alloys Compd. 2022, 916, 165445. [Google Scholar] [CrossRef]
- Wu, J.; Wu, L.; Yao, W.; Chen, Y.; Chen, Y.; Yuan, Y.; Wang, J.; Atrens, A.; Pan, F. Effect of electrolyte systems on plasma electrolytic oxidation coatings characteristics on LPSO Mg-Gd-Y-Zn alloy. Surf. Coat. Technol. 2023, 454, 129192. [Google Scholar] [CrossRef]
- Santos, J.S.; Márquez, V.; Buijnsters, J.G.; Praserthdam, S.; Praserthdam, P. Antimicrobial properties dependence on the composition and architecture of copper-alumina coatings prepared by plasma electrolytic oxidation (PEO). Appl. Surf. Sci. 2023, 607, 155072. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.; Armelin, E.; Alemán, C.; Azambuja, D.S. A synergistic combination of tetraethylorthosilicate and multiphosphonic acid offers excellent corrosion protection to AA1100 aluminum alloy. Appl. Surf. Sci. 2013, 273, 758–768. [Google Scholar] [CrossRef]
- Otani, Y.; Sasaki, S. Effects of the addition of silicon to 7075 aluminum alloy on microstructure, mechanical properties, and selective laser melting processability. Mater. Sci. Eng. A 2020, 777, 139079. [Google Scholar] [CrossRef]
- Nabavi, H.F.; Aliofkhazraei, M. Morphology, composition and electrochemical properties of bioactive-TiO2/HA on CP-Ti and Ti6Al4V substrates fabricated by alkali treatment of hybrid plasma electrolytic oxidation process (estimation of porosity from EIS results). Surf. Coat. Technol. 2019, 375, 266–291. [Google Scholar] [CrossRef]
- Pritam; Arya, A.; Sharma, A.L. Selection of best composition of Na+ ion conducting PEO-PEI blend solid polymer electrolyte based on structural, electrical, and dielectric spectroscopic analysis. Ionics 2019, 26, 745–766. [Google Scholar] [CrossRef]





| Variable | Coded | Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Time (s) | X1 | 95 | 300 | 600 | 900 | 1105 |
| Voltage (V) | X2 | 150 | 200 | 275 | 350 | 400 |
| Concentration (g/L) | X3 | 0.6 | 3.0 | 6.5 | 10.0 | 12.0 |
| Method and Materials Used | Resistance (MPa) | Ref. |
|---|---|---|
| This study | 10.7 | - |
| Welding with oxy-gas LPG/PEI glass fiber + AA2024 (PEO) | 8.5 | [7] |
| Welding with oxy-gas/PEI glass fiber + PEI glass fiber | 12.5 | [17] |
| Friction Injection Joining (F-IJ)/AA6082-T6 + PEI glass fiber | 1.1 | [21] |
| Friction Stir Spot Welding (FSSW)/AA5052 (PEO) + Polypropylene | 1.36 | [22] |
| Friction Stir Spot Welding (FSSW)/CF-PPS + CF-PPS | 2.4 | [23] |
| Source | Sum of Squares | Mean Square | F Value | Prob > F |
|---|---|---|---|---|
| Model | 24.52 | 1.75 | 0.55 | 0.7994 |
| Time | 0.38 | 0.38 | 0.12 | 0.7632 |
| Voltage | 2.94 | 2.94 | 0.93 | 0.4368 |
| Concentration | 0.061 | 0.061 | 0.019 | 0.9021 |
| Time2 | 0.26 | 0.26 | 0.083 | 0.8008 |
| Voltage2 | 0.90 | 0.90 | 0.28 | 0.6469 |
| Concentration2 | 3.96 | 3.96 | 1.25 | 0.3794 |
| Time—Voltage | 1.90 | 1.90 | 0.60 | 0.5193 |
| Time—Concentration | 2.31 | 2.31 | 0.73 | 0.4827 |
| Voltage—Concentration | 3.00 | 3.00 | 0.95 | 0.4328 |
| Time3 | 1.79 | 1.79 | 0.57 | 0.5303 |
| Voltage3 | 3.07 | 3.07 | 0.97 | 0.4285 |
| Concentration3 | 0.062 | 0.062 | 0.020 | 0.9015 |
| Time2.Concentration | 0.074 | 0.074 | 0.023 | 0.8925 |
| Time.Voltage.Concentration | 0.91 | 0.91 | 0.29 | 0.8925 |
| Error Pure | 6.33 | 3.16 | - | - |
| R2 | 79.94% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, R.R.; Marques, L.F.B.; Hein, L.R.d.O.; Botelho, E.C.; Mota, R.P. Experimental Design of the Adhesion between a PEI/Glass Fiber Composite and the AA1100 Aluminum Alloy with Oxide Coating Produced via Plasma Electrolytic Oxidation (PEO). Ceramics 2024, 7, 596-606. https://doi.org/10.3390/ceramics7020039
Lucas RR, Marques LFB, Hein LRdO, Botelho EC, Mota RP. Experimental Design of the Adhesion between a PEI/Glass Fiber Composite and the AA1100 Aluminum Alloy with Oxide Coating Produced via Plasma Electrolytic Oxidation (PEO). Ceramics. 2024; 7(2):596-606. https://doi.org/10.3390/ceramics7020039
Chicago/Turabian StyleLucas, Rafael Resende, Luis Felipe Barbosa Marques, Luis Rogerio de Oliveira Hein, Edson Cocchieri Botelho, and Rogério Pinto Mota. 2024. "Experimental Design of the Adhesion between a PEI/Glass Fiber Composite and the AA1100 Aluminum Alloy with Oxide Coating Produced via Plasma Electrolytic Oxidation (PEO)" Ceramics 7, no. 2: 596-606. https://doi.org/10.3390/ceramics7020039