Augmenting the Antitumor Efficacy of Natural Killer Cells via SynNotch Receptor Engineering for Targeted IL-12 Secretion
Abstract
:1. Introduction
2. Materials and Methods
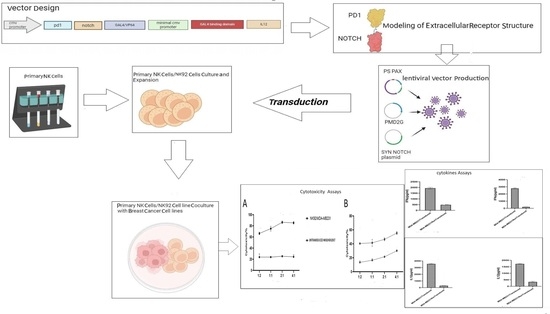
2.1. Design of SynNotch PD1 Chimeric Receptors
2.2. Modeling of Extracellular Receptor Structure
2.3. Plasmid Production
2.4. Cell Culture and Cell Lines
2.5. Production and Transduction of Lentiviruses
2.6. Assessment of Chimeric Receptor Functionality: Cytotoxicity and Cytokines Assays
2.7. Flow Cytometry Analysis
2.8. Statistical Analysis and Evaluation
3. Results
3.1. Designing and Validating the synNotch PD1 Vector
3.2. Protein Modeling
3.3. Expression of PDL1 Varies between Different Breast Cancer Cell Lines
3.4. Lentiviral Vector Synthesis and Evaluation
3.5. Interplay between PDL1 Expression, IL-12, and IFNγ Production in PD1-Syn-IL-12-NK92 Cells
3.6. Enhanced Lysis of PDL1-Positive Breast Cancer Cells by PD1-Syn-IL-12-NK Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romanski, A.; Bug, G.; Becker, S.; Kampfmann, M.; Seifried, E.; Hoelzer, D.; Ottmann, O.G.; Tonn, T. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp. Hematol. 2005, 33, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Gabriel, K.E.; Radzanowski, T.; Neyer, L.E.; Remington, J.S. Type I interferons enhance production of IFN-γ by NK cells. Immunol. Lett. 1997, 59, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Marcenaro, E.; Parolini, S.; Ferlazzo, G.; Moretta, L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008, 15, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Wendel, P.; Reindl, L.M.; Bexte, T.; Künnemeyer, L.; Särchen, V.; Albinger, N.; Mackensen, A.; Rettinger, E.; Bopp, T.; Ullrich, E. Arming immune cells for battle: A brief journey through the advancements of T and NK cell immunotherapy. Cancers 2021, 13, 1481. [Google Scholar] [CrossRef] [PubMed]
- Rafei, H.; Daher, M.; Rezvani, K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: Leveraging the power of innate immunity. Br. J. Haematol. 2021, 193, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Klein Wolterink, R.G.; Wang, J.; Bos, G.M.; Germeraad, W.T. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef]
- Siegler, E.L.; Zhu, Y.; Wang, P.; Yang, L. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell 2018, 23, 160–161. [Google Scholar] [CrossRef]
- Lu, S.-J.; Feng, Q. CAR-NK cells from engineered pluripotent stem cells: Off-the-shelf therapeutics for all patients. Stem Cells Transl. Med. 2021, 10, S10–S17. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Coyle, S.M.; Thomson, M.; Lim, W.A. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef]
- Roybal, K.T.; Williams, J.Z.; Morsut, L.; Rupp, L.J.; Kolinko, I.; Choe, J.H.; Walker, W.J.; McNally, K.A.; Lim, W.A. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 2016, 167, 419–432.e16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Yu, Z.-Y.; Cai, Y.-M.; Du, R.-R.; Cai, L. Engineering of an enhanced synthetic Notch receptor by reducing ligand-independent activation. Commun. Biol. 2020, 3, 116. [Google Scholar] [CrossRef]
- Watford, W.T.; Moriguchi, M.; Morinobu, A.; O’Shea, J.J. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003, 14, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H., Jr.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Taran, F.-A.; Wallwiener, M.; Walter, C.B.; Krämer, B.; Grischke, E.-M.; Brucker, S.Y. PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care 2016, 11, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.-C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Khalil, F.; Antonia, S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS ONE 2014, 9, e88557. [Google Scholar] [CrossRef]
- Xu, P.; Xiong, W.; Lin, Y.; Fan, L.; Pan, H.; Li, Y. Histone deacetylase 2 knockout suppresses immune escape of triple-negative breast cancer cells via downregulating PD-L1 expression. Cell Death Dis. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Janji, B.; Berchem, G. Activation of NK cells and disruption of PD-L1/PD-1 axis: Two different ways for lenalidomide to block myeloma progression. Oncotarget 2017, 8, 24031. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar] [PubMed]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A deep-learning-based platform for multi-domain protein structure and function prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef]
- Cho, D.; Campana, D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J. Lab. Med. 2009, 29, 89. [Google Scholar] [CrossRef]
- Thomas, L.M.; Peterson, M.E.; Long, E.O. Cutting edge: NK cell licensing modulates adhesion to target cells. J. Immunol. 2013, 191, 3981–3985. [Google Scholar] [CrossRef]
- Roy, S.; Barnes, P.F.; Garg, A.; Wu, S.; Cosman, D.; Vankayalapati, R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. 2008, 180, 1729–1736. [Google Scholar] [CrossRef]
- Gheidari, F.; Arefian, E.; Adegani, F.J.; Atanaki, F.F.; Soleimani, M. The miR-142 Suppresses U-87 Glioblastoma Cell Growth by Targeting EGFR Oncogenic Signaling Pathway. Iran. J. Pharm. Res. IJPR 2021, 20, 202. [Google Scholar] [PubMed]
- Shrimali, P.; Peter, M.; Singh, A.; Dalal, N.; Dakave, S.; Chiplunkar, S.V.; Tayalia, P. Efficient in situ gene delivery via PEG diacrylate matrices. Biomater. Sci. 2018, 6, 3241–3250. [Google Scholar] [CrossRef]
- Tran, J.; Kung, S.K. Lentiviral vectors mediate stable and efficient gene delivery into primary murine natural killer cells. Mol. Ther. 2007, 15, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, T.; Asgarian-Omran, H.; Memarian, A.; Shabani, M.; Sharifian, R.A.; Vossough, P.; Ansaripour, B.; Rabbani, H.; Shokri, F. Low representation of Fc receptor-like 1–5 molecules in leukemic cells from Iranian patients with acute lymphoblastic leukemia. Cancer Immunol. Immunother. 2009, 58, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Chivian, D.; Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32 (Suppl. 2), W526–W531. [Google Scholar] [CrossRef] [PubMed]
- Haron, F.; Azazi, A.; Chua, K.; Lim, Y.; Lee, P.; Chew, C. RESEARCH ARTICLE In silico structural modeling and quality assessment of Plasmodium knowlesi apical membrane antigen 1 using comparative protein models. Trop. Biomed. 2022, 39, 394–401. [Google Scholar]
- Lorenzo, M.A.; Gauna, A.N.; Herrera, J.; Bermúdez, H.; Losada, S.; Noya, O.; Serrano, M.L. In silico modeling and structural analysis of asparaginyl endopeptidase of schistosoma mansoni (Sm32): Immunological and drug target implications. Comput. Biol. Chem. 2019, 78, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.; MacArthur, M.; Thornton, J. PROCHECK: Validation of Protein-Structure Coordinates. Int. Tables Crystallogr. 2012, F, 684–687. [Google Scholar]
- Dym, O.; Eisenberg, D.; Yeates, T. ERRAT. Int. Tables Crystallogr. 2012, F, 677–683. [Google Scholar]
- Sharma, P.; Kumar, P.; Sharma, R. Natural killer cells-their role in tumour immunosurveillance. J. Clin. Diagn. Res. JCDR 2017, 11, BE01. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.H. The application of natural killer cell immunotherapy for the treatment of cancer. Front. Immunol. 2015, 6, 169989. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell–cancer cycle: Advances and new challenges in NK cell–based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Rehman, S. An overview of cancer treatment modalities. Neoplasm 2018, 1, 139–157. [Google Scholar]
- Desai, D.; Shende, P. Strategic aspects of NPY-based monoclonal antibodies for diagnosis and treatment of breast cancer. Curr. Protein Pept. Sci. 2020, 21, 1097–1102. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Giovannoni, R.; Fruci, D.; Gemignani, F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin. Cancer Biol. 2022, 79, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Lastwika, K.J.; Wilson, W., III; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non–small cell lung cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Lee, Y.K.; Koo, J.S. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J. Transl. Med. 2016, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Majidpoor, J.; Mortezaee, K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 2021, 226, 108707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bao, Y.; Meng, B.; Zheng, J.; Shi, M. From rough to precise: PD-L1 evaluation for predicting the efficacy of PD-1/PD-L1 blockades. Front. Immunol. 2022, 13, 920021. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; McKenzie, B.S.; Wilson, N.J.; de Waal Malefyt, R.; Kastelein, R.A.; Cua, D.J. IL-12 and IL-23: Master regulators of innate and adaptive immunity. Immunol. Rev. 2004, 202, 96–105. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.; Ohs, I.a.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Cohen, J. IL-12 deaths: Explanation and a puzzle. Science 1995, 270, 908. [Google Scholar] [CrossRef] [PubMed]
- Portielje, J.E.; Gratama, J.; van Ojik, H.H.; Stoter, G.; Kruit, W.H. IL-12: A promising adjuvant for cancer vaccination. Cancer Immunol. Immunother. 2003, 52, 133–144. [Google Scholar] [CrossRef]
- Koneru, M.; O’Cearbhaill, R.; Pendharkar, S.; Spriggs, D.R.; Brentjens, R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16 ecto directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Bortolanza, S.; Bunuales, M.; Otano, I.; Gonzalez-Aseguinolaza, G.; Ortiz-de-Solorzano, C.; Perez, D.; Prieto, J.; Hernandez-Alcoceba, R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther. 2009, 17, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Barnes, M.; Fewell, J.; Lewis, D.; Alvarez, R. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010, 17, 360–369. [Google Scholar] [CrossRef]
- Luo, H.; Wu, X.; Sun, R.; Su, J.; Wang, Y.; Dong, Y.; Shi, B.; Sun, Y.; Jiang, H.; Li, Z. Target-dependent expression of IL12 by synNotch receptor-engineered NK92 cells increases the antitumor activities of CAR-T cells. Front. Oncol. 2019, 9, 1448. [Google Scholar] [CrossRef]
- Prosser, M.E.; Brown, C.E.; Shami, A.F.; Forman, S.J.; Jensen, M.C. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1: CD28 chimeric receptor. Mol. Immunol. 2012, 51, 263–272. [Google Scholar] [CrossRef]
- Ankri, C.; Shamalov, K.; Horovitz-Fried, M.; Mauer, S.; Cohen, C.J. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J. Immunol. 2013, 191, 4121–4129. [Google Scholar] [CrossRef]
- Liu, X.; Ranganathan, R.; Jiang, S.; Fang, C.; Sun, J.; Kim, S.; Newick, K.; Lo, A.; June, C.H.; Zhao, Y. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016, 76, 1578–1590. [Google Scholar] [CrossRef]
- Chen, C.; Gu, Y.-M.; Zhang, F.; Zhang, Z.-C.; Zhang, Y.-T.; He, Y.-D.; Wang, L.; Zhou, N.; Tang, F.-T.; Liu, H.-J. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer. Oncoimmunology 2021, 10, 1901434. [Google Scholar] [CrossRef]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the bridge between innate and adaptive immunity for cancer immunotherapy: Focus on γδ T and NK cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Esser, R.; Priesner, C.; Suerth, J.D.; Wels, W.S.; Grez, M.; Kloess, S.; Arseniev, L.; Koehl, U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015, 6, 129355. [Google Scholar] [CrossRef] [PubMed]
- Kloess, S.; Kretschmer, A.; Stahl, L.; Fricke, S.; Koehl, U. CAR-expressing natural killer cells for cancer retargeting. Transfus. Med. Hemotherapy 2019, 46, 4–13. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadnia, A.; Mohammadi, S.; Yamchi, A.; Kalani, M.R.; Farazmandfar, T.; Khosravi, A.; Memarian, A. Augmenting the Antitumor Efficacy of Natural Killer Cells via SynNotch Receptor Engineering for Targeted IL-12 Secretion. Curr. Issues Mol. Biol. 2024, 46, 2931-2945. https://doi.org/10.3390/cimb46040183
Ahmadnia A, Mohammadi S, Yamchi A, Kalani MR, Farazmandfar T, Khosravi A, Memarian A. Augmenting the Antitumor Efficacy of Natural Killer Cells via SynNotch Receptor Engineering for Targeted IL-12 Secretion. Current Issues in Molecular Biology. 2024; 46(4):2931-2945. https://doi.org/10.3390/cimb46040183
Chicago/Turabian StyleAhmadnia, Ali, Saeed Mohammadi, Ahad Yamchi, Mohamad Reza Kalani, Touraj Farazmandfar, Ayyoub Khosravi, and Ali Memarian. 2024. "Augmenting the Antitumor Efficacy of Natural Killer Cells via SynNotch Receptor Engineering for Targeted IL-12 Secretion" Current Issues in Molecular Biology 46, no. 4: 2931-2945. https://doi.org/10.3390/cimb46040183