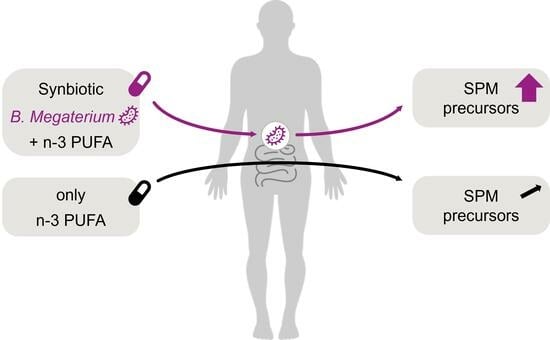
Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
- (1)
- Relevant history or presence of any severe medical disorder, potentially interfering with this study (e.g., malabsorption, chronic gastro-intestinal diseases, heavy depression, diabetes, acute cancers within the last 3 years except basal cell carcinoma of the skin, etc.);
- (2)
- Subject under prescription for medication or taking dietary supplements possibly interfering with this study (such as n-3 PUFA, probiotics, anti-spasmodic, laxatives and anti-diarrheic drugs or other digestive auxiliaries, use of PPIs, bismuth salts and/or H2-antagonists, fibers, chronic intake of NSAR or acetylsalicylic acid, glucocorticoids, etc.) within 2 weeks before study start or during the study;
- (3)
- Intake of antibiotics in the last 2 months and laxatives in the last 2 weeks.
2.3. Study Products
2.4. Methods for Samples and Data Collection
Blood Sampling
2.5. Analysis of Lipid Mediators, Omega-3 Index, and n-3 PUFA
2.5.1. Analysis of Lipid Mediators in Plasma
2.5.2. Omega-3 Index and Total n-3 PUFA in Plasma
2.6. Inflammatory Biomarkers in Blood
2.7. Statistical Analysis
3. Results
3.1. Study Collective
3.2. Primary Efficacy Endpoint: Significant Increase in the Sum of 18-HEPE and 5-HEPE by SynΩ3
3.3. Treatment Effects on Selected Lipid Mediators
3.4. Modulation of n-3 PUFA Levels in Blood Compartments
3.5. EPA and DHA as Free Fatty Acids in Plasma
3.6. Evaluation of Cholesterols and Triglycerides
3.7. Evaluation of TNF-α and hsCRP
3.8. Safety and Tolerability
4. Discussion
5. Conclusions
6. Patent Applications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, J.H.; Lim, H.; Lim, S. The Potential Cardiometabolic Effects of Long-Chain omega-3 Polyunsaturated Fatty Acids: Recent Updates and Controversies. Adv. Nutr. 2023, 14, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77917 Individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Kuno, T.; Morita, S.X.; Slipczuk, L.; Takagi, H.; Briasoulis, A.; Latib, A.; Bangalore, S.; Heffron, S.P. Eicosapentaenoic Acid for Cardiovascular Events Reduction- Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Cardiol. 2022, 80, 416–422. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yu, H.; Fang, J.; Qi, Z.; Pei, S.; Yan, B.; Liu, R.; Wang, Q.; Szeto, I.M.; Liu, B.; et al. The effect of n-3 polyunsaturated fatty acid supplementation on cognitive function outcomes in the elderly depends on the baseline omega-3 index. Food Funct. 2023, 14, 9506–9517. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Liu, M.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Felger, J.C.; Ziegler, T.R.; Fava, M.; Mischoulon, D.; Rapaport, M.H. Clinical response to EPA supplementation in patients with major depressive disorder is associated with higher plasma concentrations of pro-resolving lipid mediators. Neuropsychopharmacology 2023, 48, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Jordan, P.M.; Gerstmeier, J.; Pace, S.; Bilancia, R.; Rao, Z.; Börner, F.; Miek, L.; Gutiérrez-Gutiérrez, Ó.; Arakandy, V.; Rossi, A.; et al. Staphylococcus aureus-Derived alpha-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution. Cell Rep. 2020, 33, 108247. [Google Scholar] [CrossRef]
- Lopez-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; Garcia-Alonso, V.; Lopategi, A.; Titos, E.; Claria, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Zhang, X.; Huang, J.; He, J.; Peng, L.; Ye, C.; Wang, Y.; Xue, F.; Ai, D.; et al. Metabolic profiling of murine plasma reveals eicosapentaenoic acid metabolites protecting against endothelial activation and atherosclerosis. Br. J. Pharmacol. 2018, 175, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Sano, M.; Isobe, Y.; Fukuda, K.; Kang, J.X.; Arai, H.; Arita, M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J. Exp. Med. 2014, 211, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, M.; Liu, Y.; Suo, X.; Fan, G.; Yang, X. 5-HEPE reduces obesity and insulin resistance by promoting adipose tissue browning through GPR119/AMPK/PGC1alpha activation. Life Sci. 2023, 323, 121703. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Pochard, C.; Coquenlorge, S.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Meurette, G.; Freyssinet, M.; Neunlist, M.; Rolli-Derkinderen, M. Defects in 15-HETE Production and Control of Epithelial Permeability by Human Enteric Glial Cells From Patients With Crohn’s Disease. Gastroenterology 2016, 150, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am. J. Clin. Nutr. 2015, 102, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Dalli, J.; Norling, L.V.; Colas, R.A.; Perretti, M.; Serhan, C.N. Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J. Immunol. 2016, 197, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; de la Rosa, X.; Muller, A.; Mayr, M.; Gonzalez Santos, F.; Gracia Banzo, R.; Rizo, J.M. PCOS: A Chronic Disease That Fails to Produce Adequately Specialized Pro-Resolving Lipid Mediators (SPMs). Biomedicines 2022, 10, 456. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102219. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Gasper, W.J.; Khetani, S.A.; Zahner, G.J.; Hills, N.K.; Mitchell, P.T.; Sansbury, B.E.; Conte, M.S.; Spite, M.; Grenon, S.M. Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial). J. Surg. Res. 2019, 238, 164–174. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2021, 316, 90–98. [Google Scholar] [CrossRef]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050. [Google Scholar] [CrossRef] [PubMed]
- Irun, P.; Carrera-Lasfuentes, P.; Sanchez-Luengo, M.; Belio, U.; Domper-Arnal, M.J.; Higuera, G.A.; Hawkins, M.; de la Rosa, X.; Lanas, A. Pharmacokinetics and Changes in Lipid Mediator Profiling after Consumption of Specialized Pro-Resolving Lipid-Mediator-Enriched Marine Oil in Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 16143. [Google Scholar] [CrossRef]
- Speckmann, B.; Kleinbolting, J.; Borner, F.; Jordan, P.M.; Werz, O.; Pelzer, S.; Tom Dieck, H.; Wagner, T.; Schon, C. Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients 2022, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.S.; Zahner, G.J.; Gasper, W.J.; Harris, W.S.; Conte, M.S.; Hills, N.K.; Grenon, S.M. Relationship between the omega-3 index and specialized pro-resolving lipid mediators in patients with peripheral arterial disease taking fish oil supplements. J. Clin. Lipidol. 2017, 11, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef]
- Werner, M.; Jordan, P.M.; Romp, E.; Czapka, A.; Rao, Z.; Kretzer, C.; Koeberle, A.; Garscha, U.; Pace, S.; Claesson, H.E.; et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019, 33, 6140–6153. [Google Scholar] [CrossRef]
- Koba, S.; Takao, T.; Shimizu, F.; Ogawa, M.; Ishii, Y.; Yokota, Y.; Furuyama, F.; Tsunoda, F.; Shoji, M.; Harris, W.S.; et al. Comparison of plasma levels of different species of trans fatty acids in Japanese male patients with acute coronary syndrome versus healthy men. Atherosclerosis 2019, 284, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.H.; Wei, S.; Helvig, C.; Falck, J.R.; Belosludtsev, Y.; Truan, G.; Graham-Lorence, S.E.; Peterson, J.A. The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450BM-3. J. Biol. Chem. 1996, 271, 22663–22671. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Serhan, C.N. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Ehring, E.; Hu, J.; Rodriguez Mateos, A. Exploring substrate-microbe interactions: A metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes 2024, 16, 2305716. [Google Scholar] [CrossRef]
- Murphy, R.C. Specialized pro-resolving mediators: Do they circulate in plasma? J. Lipid Res. 2015, 56, 1641–1642. [Google Scholar] [CrossRef]
- Miyoshi, T.; Naoe, S.; Wakabayashi, H.; Yano, T.; Mori, T.; Kanda, S.; Arita, M.; Ito, H. Enhanced Production of EPA-Derived Anti-Inflammatory Metabolites after Oral Administration of a Novel Self-Emulsifying Highly Purified EPA Ethyl Ester Formulation (MND-2119). J. Atheroscler. Thromb. 2023, 30, 1927–1949. [Google Scholar] [CrossRef]








| Variables | Study Population Mean (± 95% CI) |
|---|---|
| Age (years) | 54.5 (51.9–57.1) |
| BMI (kg/m2) | 25.7 (24.8–26.6) |
| Cholesterol (mg/dL) | 219.7 (209.7–229.6) |
| LDL-cholesterol (mg/dL) | 146.8 (136.5–157.1) |
| GPT (U/L) | 26.7 (24.3–29.1) |
| GOT (U/L) | 26.1 (24.7–27.5) |
| hsCRP (mg/L) | 1.9 (1.4–2.4) |
| Systolic blood pressure (mmHg) | 126.2 (122.5–129.9) |
| Diastolic blood pressure (mmHg) | 79.2 (76.8–81.6) |
| Omega-3 index in erythrocytes (%) | 4.9 (4.7–5.1) |
| AGE in skin | 31.9% no risk 55.6% risk group 1 5.6% risk group 2 6.9% risk group 3 |
| Lipid Metabolites | Assessment Points | Placebo (n = 24) Mean (95% CI) | SynΩ3 (n = 23) Mean (95% CI) | Fish Oil (n = 25) Mean (95% CI) | Difference in Mean Change SynΩ3 vs. Placebo | p-Value § | Difference in Mean Change SynΩ3 vs. Fish Oil | p-Value # | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA metabolites | 5-HETE [pg/mL] | Baseline | 196.8 | (160.9, 232.6) | 178.4 | (156.4, 200.5) | 208.4 | (171.9, 244.9) | ||||
| 2 days | 201.8 | (166., 237.5) | 184.8 | (154.5, 215.2) | 203.3 | (164.8, 241.9) | 1.4 | 0.3580 | 11.5 | 0.4712 | ||
| 4 weeks | 202.9 | (173.8, 231.9) | 178.2 | (150.7, 205.6) | 187.5 | (153.4, 221.7) | −6.3 | 0.8670 | 20.7 | 0.2357 | ||
| 15-HETE ** [pg/mL] | Baseline | 210.2 | (180.3, 240.) | 188.4 | (163.7, 213.2) | 196.5 | (170.1, 222.9) | |||||
| 2 days | 196.6 | (170.6, 222.5) | 193.9 | (167., 220.8) | 196.5 | (168.3, 224.8) | 19.1 | 0.3949 | 5.5 | 0.3491 * | ||
| 4 weeks | 209.6 | (179.7, 239.5) | 186.5 | (151.9, 221.1) | 181.4 | (154.8, 208.0) | −1.3 | 0.5728 * | 13.2 | 0.4186 | ||
| EPA metabolites | 18-HEPE+5-HEPE [pg/mL] | Baseline | 214.0 | (164.9, 263.1) | 185.8 | (152.4, 219.3) | 204.8 | (164.5, 245.0) | ||||
| 2 days | 204.3 | (160.0, 248.7) | 620.7 | (432.7, 808.8) | 254.1 | (211.4, 296.8) | 444.6 | <0.0001 * | 385.6 | <0.0001 * | ||
| 4 weeks | 216.4 | (187.6, 245.1) | 642.5 | (420.9, 864.1) | 242.4 | (202.2, 282.7) | 454.3 | <0.0001 * | 419.1 | 0.0003 * | ||
| 18-HEPE [pg/mL] | Baseline | 109.0 | (78.1, 139.8) | 97.0 | (75.4, 118.6) | 105.1 | (75.8, 134.5) | |||||
| 2 days | 99.8 | (77.5, 122.1) | 395.2 | (218.5, 571.9) | 142.3 | (105.5, 179.1) | 307.4 | <0.0001 * | 261.0 | 0.0003 * | ||
| 4 weeks | 112.0 | (96.3, 127.6) | 446.2 | (247.7, 644.6) | 132.2 | (105.2, 159.2) | 346.2 | 0.0007 * | 322.1 | 0.0032 * | ||
| 5-HEPE [pg/mL] | Baseline | 105.0 | (81.6, 128.5) | 88.8 | (70.5, 107.1) | 99.6 | (80.7, 118.5) | |||||
| 2 days | 104.5 | (79.5, 129.6) | 225.5 | (177.3, 273.8) | 111.8 | (97.6, 125.9) | 137.2 | <0.0001 * | 124.5 | <0.0001 | ||
| 4 weeks | 104.4 | (85.1, 123.7) | 196.3 | (145.8, 246.9) | 110.3 | (98.7, 130.8) | 108.1 | 0.0001 * | 96.8 | 0.0005 * | ||
| 15-HEPE [pg/mL] | Baseline | 33.9 | (21.9, 45.9) | 27.6 | (22.3, 32.9) | 31.3 | (21.9, 40.7) | |||||
| 2 days | 30.3 | (24.1, 36.5) | 49.6 | (38.1, 61.0) | 41.2 | (27.8, 54.7) | 25.6 | 0.0002 * | 12.1 | 0.1351 * | ||
| 4 weeks | 33.5 | (26.6, 40.3) | 46.4 | (31.0, 61.7) | 37.2 | (27.4, 47.0) | 19.2 | 0.0924 * | 12.9 | 0.3470 * | ||
| DHA metabolites | 4-HDHA [pg/mL] | Baseline | 55.7 | (45.3, 66.2) | 53.5 | (44.7, 62.4) | 50.1 | (40.5, 59.7) | ||||
| 2 days | 51.3 | (43.5, 59.1) | 76.3 | (64.8, 87.8) | 55.4 | (44.1, 66.8) | 27.2 | 0.0004 * | 17.4 | 0.0048 | ||
| 4 weeks | 53.5 | (45.5, 61.6) | 74.3 | (62.9, 85.7) | 60.8 | (50.3, 71.2) | 22.9 | 0.0014 | 10.1 | 0.1127 | ||
| 10-HDHA [pg/mL] | Baseline | 28.0 | (22.5, 33.5) | 31.6 | (25.6, 37.5) | 33.9 | (21.2, 46.6) | |||||
| 2 days | 30.0 | (24.1, 35.9) | 43.6 | (35.4, 51.9) | 37.7 | (23.5, 51.9) | 10.1 | 0.0319 * | 8.3 | 0.0449 | ||
| 4 weeks | 28.4 | (23.6, 33.3) | 48.7 | (38.4, 59.1) | 36.1 | (28.7, 43.5) | 16.8 | 0.0096 * | 15.0 | 0.0516 * | ||
| 17-HDHA [pg/mL] | Baseline | 80.3 | (62.6, 98.0) | 89.5 | (71.2, 107.9) | 122.3 | (68.8, 175.8) | |||||
| 2 days | 86.5 | (70.8, 102.1) | 146.3 | (109.9, 182.6) | 125.6 | (61.2, 190.0) | 50.7 | 0.0144 * | 53.5 | 0.0246 * | ||
| 4 weeks | 107.1 | (82.3, 132.0) | 100.4 | (75.6, 125.2) | 125.3 | (87.0, 163.7) | −15.9 | 0.2770 * | 7.9 | 0.9913 * | ||
| Lipid Status | Assessment Points | Placebo (n = 24) Mean (95% CI) | SynΩ3 (n = 23) Mean (95% CI) | Fish Oil (n = 25) Mean (95% CI) | Difference in Mean Change SynΩ3 vs. Placebo | p-Value § | Difference in Mean Change SynΩ3 vs. Fish Oil | p-Value # | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cholesterol [mg/dL] | Baseline | 210.3 | (192.5, 228.0) | 228.7 | (211.8, 245.7) | 224.4 | (207.7, 241.2) | ||||
| 4 weeks | 210.3 | (193.2, 227.3) | 224.4 | (205.9, 243.0) | 219.8 | (203.2, 236.4) | −4.3 | 0.6624 | 0.3 | 0.9414 | |
| LDL [mg/dL] | Baseline | 137.0 | (118.7, 155.3) | 156.1 | (140.6, 171.7) | 149.7 | (133.4, 166.1) | ||||
| 4 weeks | 136.9 | (118.7, 155.1) | 157.8 | (140.3, 175.3) | 148.6 | (131.9, 165.3) | 1.8 | 0.6233 | 2.8 | 0.5569 | |
| HDL [mg/dL] | Baseline | 60.4 | (54.2, 66.6) | 58.5 | (52.8, 64.2) | 60.9 | (55.3, 66.6) | ||||
| 4 weeks | 63.5 | (56.4, 70.7) | 57.9 | (51.5, 64.3) | 61.7 | (55.5, 68.0) | −3.8 | 0.0331 | −1.4 | 0.3517 | |
| Triglycerides [mg/dL] | Baseline | 105.3 | (74.9, 135.6) | 114.3 | (97.2, 131.3) | 104.2 | (82.2, 126.3) | ||||
| 4 weeks | 113.1 | (82.3, 144.0) | 102.0 | (81.9, 122.2) | 92.2 | (74.8, 109.7) | −20.1 | 0.1034* | −0.3 | 0.4387* | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speckmann, B.; Wagner, T.; Jordan, P.M.; Werz, O.; Wilhelm, M.; tom Dieck, H.; Schön, C. Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study. Nutrients 2024, 16, 1354. https://doi.org/10.3390/nu16091354
Speckmann B, Wagner T, Jordan PM, Werz O, Wilhelm M, tom Dieck H, Schön C. Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study. Nutrients. 2024; 16(9):1354. https://doi.org/10.3390/nu16091354
Chicago/Turabian StyleSpeckmann, Bodo, Tanja Wagner, Paul M. Jordan, Oliver Werz, Manfred Wilhelm, Heike tom Dieck, and Christiane Schön. 2024. "Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study" Nutrients 16, no. 9: 1354. https://doi.org/10.3390/nu16091354