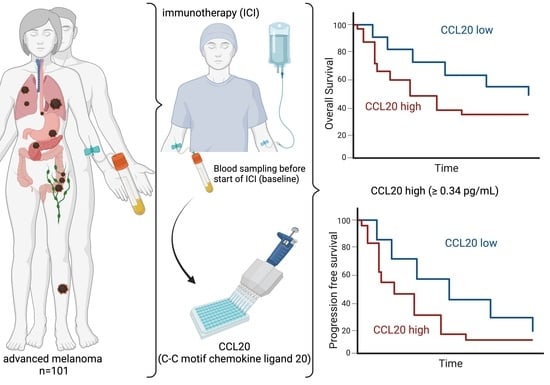
High Serum Levels of CCL20 Are Associated with Recurrence and Unfavorable Overall Survival in Advanced Melanoma Patients Receiving Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Peripheral Blood Samples
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for CCL20 Levels
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Martin, A.R.; Ronco, C.; Rocchi, S.; Benhida, R. Metastatic Melanoma: Insights Into the Evolution of the Treatments and Future Challenges. Med. Res. Rev. 2017, 37, 98–148. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, A.; Eggermont, A.M.M.; Robert, C.; Dummer, R.; Ugurel, S.; Livingstone, E.; Ascierto, P.A.; Long, G.V.; Schadendorf, D.; Zimmer, L. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur. J. Cancer 2021, 155, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.; Ademuyiwa, F.O.; Cao, Z.A.; Chen, H.X.; Ferris, R.L.; Goldberg, S.B.; Hellmann, M.D.; Mehra, R.; Rhee, I.; Park, J.C.; et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors with chemotherapy. J. Immunother. Cancer 2023, 11, e005921. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabieres, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Pantel, K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Su, J.; Zhou, Y.; Zhang, C.; Wang, Y. CCL20/CCR6 promotes cell proliferation and metastasis in laryngeal cancer by activating p38 pathway. Biomed. Pharmacother. 2017, 85, 486–492. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 5186. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Wang, S.; Sheng, Y.; Wu, Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): Mechanisms and communication networks in breast cancer progression. Bioengineered 2021, 12, 6923–6934. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Han, J.; Wu, H.; Liu, Z. Proteasome-dependent senescent tumor cells mediate immunosuppression through CCL20 secretion and M2 polarization in pancreatic ductal adenocarcinoma. Front. Immunol. 2023, 14, 1216376. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Li, Y.; Yang, X.; Han, M.; Fan, Q. Construction of a CCL20-centered circadian-signature based prognostic model in cervical cancer. Cancer Cell Int. 2023, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhu, T.; Wang, J.; Jiang, R.; Shu, A.; Zhang, Z.; Zhang, P.; Feng, X.; Zhao, L. miR-22 promotes immunosuppression via activating JAK/STAT3 signaling in cutaneous squamous cell carcinoma. Carcinogenesis 2023, 44, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.Z.; Fu, Z.Q.; Gong, H. Chemokine ligand 20 enhances progression of hepatocellular carcinoma via epithelial-mesenchymal transition. World J. Gastroenterol. 2015, 21, 475–483. [Google Scholar] [CrossRef]
- Martin-Garcia, D.; Silva-Vilches, C.; Will, R.; Enk, A.H.; Lonsdorf, A.S. Tumor-derived CCL20 affects B16 melanoma growth in mice. J. Dermatol. Sci. 2020, 97, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, R.; Gutierrez-Gonzalez, A.; Gutierrez-Seijo, A.; Sanchez-Gregorio, S.; Garcia-Gimenez, J.; Mercader, E.; Marquez-Rodas, I.; Aviles, J.A.; Relloso, M.; Sanchez-Mateos, P. CCL20 Expression by Tumor-Associated Macrophages Predicts Progression of Human Primary Cutaneous Melanoma. Cancer Immunol. Res. 2018, 6, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert. Rev. Anticancer. Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Harrison, E.; Drake, T.; Pius, R. Finalfit: Quickly Create Elegant Regression Results Tables and Plots when Modelling. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=finalfit (accessed on 21 April 2024).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.9. Available online: https://CRAN.R-project.org/package=survminer (accessed on 21 April 2024).
- Therneau, T. A Package for Survival Analysis in R, R Package Version 3.5-7. Available online: https://CRAN.R-project.org/package=survival (accessed on 21 April 2024).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hothorn, T.; Lausen, B. Maxstat: Maximally Selected Rank Statistics. R Package Version 0.7-25. Available online: https://CRAN.R-project.org/package=maxstat (accessed on 21 April 2024).
- Wang, D.; Yuan, W.; Wang, Y.; Wu, Q.; Yang, L.; Li, F.; Chen, X.; Zhang, Z.; Yu, W.; Maimela, N.R.; et al. Serum CCL20 combined with IL-17A as early diagnostic and prognostic biomarkers for human colorectal cancer. J. Transl. Med. 2019, 17, 253. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Kahler, K.C.; Hassel, J.C.; Heinzerling, L.; Loquai, C.; Mossner, R.; Ugurel, S.; Zimmer, L.; Gutzmer, R.; Cutaneous Side Effects Committee of the Work Group Dermatological, O. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J. Dtsch. Dermatol. Ges. 2016, 14, 662–681. [Google Scholar] [CrossRef]
- Amend, K.L.; Elder, J.T.; Tomsho, L.P.; Bonner, J.D.; Johnson, T.M.; Schwartz, J.; Berwick, M.; Gruber, S.B. EGF gene polymorphism and the risk of incident primary melanoma. Cancer Res. 2004, 64, 2668–2672. [Google Scholar] [CrossRef] [PubMed]
- Hippe, A.; Braun, S.A.; Olah, P.; Gerber, P.A.; Schorr, A.; Seeliger, S.; Holtz, S.; Jannasch, K.; Pivarcsi, A.; Buhren, B.; et al. EGFR/Ras-induced CCL20 production modulates the tumour microenvironment. Br. J. Cancer 2020, 123, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Fenouille, N.; Tichet, M.; Dufies, M.; Pottier, A.; Mogha, A.; Soo, J.K.; Rocchi, S.; Mallavialle, A.; Galibert, M.D.; Khammari, A.; et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS ONE 2012, 7, e40378. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Mosca, P.J.; Robertson, G.P. The Akt signaling pathway: An emerging therapeutic target in malignant melanoma. Cancer Biol. Ther. 2011, 12, 1032–1049. [Google Scholar] [CrossRef]
- Tompa, M.; Kalovits, F.; Nagy, A.; Kalman, B. Contribution of the Wnt Pathway to Defining Biology of Glioblastoma. Neuromolecular Med. 2018, 20, 437–451. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Fodde, R.; Brabletz, T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007, 19, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Rutihinda, C.; Haroun, R.; Saidi, N.E.; Ordonez, J.P.; Naasri, S.; Levesque, D.; Boisvert, F.M.; Fortier, P.H.; Belzile, M.; Fradet, L.; et al. Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy. Cancer Immunol. Immunother. 2023, 72, 1089–1102. [Google Scholar] [CrossRef] [PubMed]



| Total N (%) | N (%) | ||
|---|---|---|---|
| Sex | 101 (100.0) | Male | 69 (68.3) |
| Female | 32 (31.7) | ||
| Age group | 101 (100.0) | <65 | 39 (38.6) |
| ≥65 | 62 (61.4) | ||
| ECOG | 101 (100.0) | 0 | 51 (50.5) |
| 1 | 34 (33.7) | ||
| 2 | 11 (10.9) | ||
| 3 | 5 (5.0) | ||
| Primary melanoma site | 101 (100.0) | Cutaneous | 73 (72.3) |
| Mucosal | 8 (7.9) | ||
| Uveal | 20 (19.8) | ||
| AJCC Stage | 101 (100.0) | Stage III | 8 (7.9) |
| Stage IV | 93 (92.1) | ||
| T | 101 (100.0) | T0 | 19 (18.8) |
| T1 | 7 (6.9) | ||
| T2 | 11 (10.9) | ||
| T3 | 11 (10.9) | ||
| T4 | 33 (32.7) | ||
| Tx | 20 (19.8) | ||
| N | 101 (100.0) | N0 | 41 (40.6) |
| N1 | 23 (22.8) | ||
| N2 | 14 (13.9) | ||
| N3 | 18 (17.8) | ||
| Nx | 5 (5.0) | ||
| M | 101 (100.0) | M0 | 11 (10.9) |
| M1 | 90 (89.1) | ||
| Baseline therapy | 101 (100.0) | Ipilimumab + Nivolumab | 64 (63.4) |
| Nivolumab | 12 (11.9) | ||
| Pembrolizumab | 16 (15.8) | ||
| Tebentafusp | 8 (7.9) | ||
| Cemiplimab | 1 (1.0) |
| Total N (%) | Low CCL20 < 0.34 pg/mL N (%) | High CCL20 ≥ 0.34 pg/mL N (%) | p-Value (Fisher’s Exact Test) | ||
|---|---|---|---|---|---|
| Sex | 101 (100.0) | Male | 35 (64.8) | 34 (72.3) | 0.521 |
| Female | 19 (35.2) | 13 (27.7) | |||
| Age group | 101 (100.0) | <65 | 15 (27.8) | 24 (51.1) | 0.024 |
| ≥65 | 39 (72.2) | 23 (48.9) | |||
| ECOG | 101 (100.0) | 0 | 32 (59.3) | 19 (40.4) | 0.241 |
| 1 | 14 (25.9) | 20 (42.6) | |||
| 2 | 5 (9.3) | 6 (12.8) | |||
| 3 | 3 (5.6) | 2 (4.3) | |||
| Primary melanoma site | 101 (100.0) | Cutaneous | 43 (79.6) | 30 (63.8) | 0.060 |
| Mucosal | 5 (9.3) | 3 (6.4) | |||
| Uveal | 6 (11.1) | 14 (29.8) | |||
| AJCC Stage | 101 (100.0) | Stage III | 5 (9.3) | 3 (6.4) | 0.721 |
| Stage IV | 49 (90.7) | 44 (93.6) | |||
| T | 101 (100.0) | T0 | 9 (16.7) | 10 (21.3) | 0.782 |
| T1 | 4 (7.4) | 3 (6.4) | |||
| T2 | 8 (14.8) | 3 (6.4) | |||
| T3 | 6 (11.1) | 5 (10.6) | |||
| T4 | 18 (33.3) | 15 (31.9) | |||
| Tx | 9 (16.7) | 11 (23.4) | |||
| N | 101 (100.0) | N0 | 19 (35.2) | 22 (46.8) | 0.600 |
| N1 | 12 (22.2) | 11 (23.4) | |||
| N2 | 9 (16.7) | 5 (10.6) | |||
| N3 | 10 (18.5) | 8 (17.0) | |||
| Nx | 4 (7.4) | 1 (2.1) | |||
| M | 101 (100.0) | M0 | 7 (13.0) | 4 (8.5) | 0.537 |
| M1 | 47 (87.0) | 43 (91.5) | |||
| Baseline Therapy | 101 (100.0) | Ipilimumab + Nivolumab | 35 (64.8) | 29 (61.7) | 0.686 |
| Nivolumab | 6 (11.1) | 6 (12.8) | |||
| Pembrolizumab | 10 (18.5) | 6 (12.8) | |||
| Tebentafusp | 3 (5.6) | 5 (10.6) | |||
| Cemiplimab | 0 (0.0) | 1 (2.1) | |||
| Progressive Disease | 101 (100.0) | No | 19 (35.2) | 7 (14.9) | 0.024 |
| Yes | 35 (64.8) | 40 (85.1) |
| Total N (%) | Low CCL20 < 0.34 pg/mL | High CCL20 ≥ 0.34 pg/mL | p-Value | ||
|---|---|---|---|---|---|
| LDH [U/L] | 98 (97.0) | Median (IQR) | 258 (220.75 to 322.50) | 329 (262.25 to 575.00) | 0.004 (a) |
| S100 [µg/L] | 90 (89.1) | Median (IQR) | 0.147 (0.06 to 0.48) | 0.257 (0.09 to 1.16) | 0.049 (a) |
| D-Dimers [mg/L] | 83 (82.2) | Median (IQR) | 0.915 (0.58 to 1.73) | 1.170 (0.47 to 3.30) | 0.489 (a) |
| CRP [mg/L] | 96 (95.0) | Median (IQR) | 5 (2.50 to 25.00) | 10 (5.00 to 34.00) | 0.076 (a) |
| Neutrophil count [×109/L] | 93 (92.1) | Median (IQR) | 4.830 (3.92 to 5.88) | 5.275 (4.28 to 7.46) | 0.278 (a) |
| Lymphocyte count [×109/L] | 93 (92.1) | Mean (SD) | 1.486 (0.61) | 1.330 (0.54) | 0.192 (b) |
| Neutrophil/Lymphocyte ratio (NLR) | 93 (92.1) | Median (IQR) | 3.199 (2.65 to 5.31) | 3.854 (2.86 to 6.69) | 0.167 (a) |
| Progression-Free Survival | N (%) | HR (Univariable) (95% CI) | HR (Multivariable) (95% CI) | |
|---|---|---|---|---|
| Sex | Male | 69 (68.3) | - | - |
| Female | 32 (31.7) | 0.67 (0.40–1.12, p = 0.129) | - | |
| Age group | <65 | 39 (38.6) | - | - |
| ≥65 | 62 (61.4) | 0.86 (0.54–1.36, p = 0.508) | - | |
| ECOG | 0 | 51 (50.5) | - | - |
| 1 | 34 (33.7) | 1.34 (0.81–2.24, p = 0.257) | 1.54 (0.89–2.67, p = 0.127) | |
| 2 | 11 (10.9) | 1.96 (0.97–3.98, p = 0.062) | 2.18 (0.98–4.83, p = 0.056) | |
| 3 | 5 (5.0) | 7.03 (2.63–18.80, p < 0.001) | 9.48(3.36–26.75, p < 0.001) | |
| AJCC Stage | Stage III | 8 (7.9) | - | - |
| Stage IV | 93 (92.1) | 1.70 (0.69–4.22, p = 0.252) | - | |
| Primary melanoma site | Cutaneous | 73 (72.3) | - | - |
| Mucosal | 8 (7.9) | 1.80 (0.81–4.00, p = 0.151) | - | |
| Uveal | 20 (19.8) | 1.57 (0.90–2.72, p = 0.111) | - | |
| Baseline therapy | Ipilimumab + Nivolumab | 64 (63.4) | - | - |
| Nivolumab | 12 (11.9) | 1.12 (0.56–2.22, p = 0.752) | - | |
| Pembrolizumab | 16 (15.8) | 0.91 (0.48–1.71, p = 0.760) | - | |
| Tebentafusp | 8 (7.9) | 1.37 (0.59–3.22, p = 0.466) | - | |
| Cemiplimab | 1 (1.0) | 7.24 (0.95–55.01, p = 0.056) | - | |
| CCL20 group | Low-CCL20 < 0.34 pg/mL | 54 (53.5) | - | - |
| High-CCL20 ≥ 0.34 pg/mL | 47 (46.5) | 1.98 (1.25–3.12, p = 0.004) | 1.99 (1.21–3.29, p = 0.007) | |
| LDH | Not elevated | 28 (28.6) | - | - |
| Elevated | 70 (71.4) | 0.91 (0.55–1.51, p = 0.720) | - | |
| S100 | Not elevated | 40 (44.4) | - | - |
| Elevated | 50 (55.6) | 1.74 (1.05–2.86, p = 0.030) | 1.74 (1.05–2.90, p = 0.033) |
| Overall Survival | N (%) | HR (Univariable) (95% CI) | HR (Multivariable) (95% CI) | |
|---|---|---|---|---|
| Sex | Male | 69 (68.3) | - | - |
| Female | 32 (31.7) | 0.71 (0.36–1.40, p = 0.317) | - | |
| Age group | <65 | 39 (38.6) | - | - |
| ≥65 | 62 (61.4) | 1.08 (0.59–1.98, p = 0.811) | - | |
| ECOG | 0 | 51 (50.5) | - | - |
| 1 | 34 (33.7) | 4.20 (1.95–9.07, p < 0.001) | 4.58(1.97–10.60, p < 0.001) | |
| 2 | 11 (10.9) | 7.87 (3.24–19.11, p < 0.001) | 7.46(2.75–20.24, p < 0.001) | |
| 3 | 5 (5.0) | 16.14(5.26–49.53, p < 0.001) | 15.36(4.75–49.67, p < 0.001) | |
| AJCC | Stage III | 8 (7.9) | - | - |
| Stage IV | 93 (92.1) | 2.03 (0.49–8.40, p = 0.327) | - | |
| Primary melanoma site | Cutaneous | 73 (72.3) | - | - |
| Mucosal | 8 (7.9) | 1.77 (0.68–4.58, p = 0.241) | - | |
| Uveal | 20 (19.8) | 1.48 (0.72–3.06, p = 0.283) | - | |
| Baseline therapy | Ipilimumab + Nivolumab | 64 (63.4) | - | - |
| Nivolumab | 12 (11.9) | 0.72 (0.25–2.05, p = 0.537) | - | |
| Pembrolizumab | 16 (15.8) | 1.08 (0.50–2.37, p = 0.838) | - | |
| Tebentafusp | 8 (7.9) | 0.57 (0.14–2.40, p = 0.444) | - | |
| Cemiplimab | 1 (1.0) | 5.06 (0.67–38.26, p = 0.117) | - | |
| CCL20 group | Low-CCL20 < 0.34 pg/mL | 54 (53.5) | - | - |
| High-CCL20 ≥ 0.34 pg/mL | 47 (46.5) | 1.85 (1.02–3.37, p = 0.043) | 1.46 (0.77–2.75, p = 0.244) | |
| LDH | Not elevated | 28 (28.6) | - | - |
| Elevated | 70 (71.4) | 1.16 (0.58–2.31, p = 0.680) | - | |
| S100 | Not elevated | 40 (44.4) | - | - |
| Elevated | 50 (55.6) | 1.99 (1.02–3.88, p = 0.043) | 1.78 (0.90–3.54, p = 0.098) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kött, J.; Hoehne, I.L.; Heidrich, I.; Zimmermann, N.; Reese, K.-L.; Zell, T.; Geidel, G.; Rünger, A.; Schneider, S.W.; Pantel, K.; et al. High Serum Levels of CCL20 Are Associated with Recurrence and Unfavorable Overall Survival in Advanced Melanoma Patients Receiving Immunotherapy. Cancers 2024, 16, 1737. https://doi.org/10.3390/cancers16091737
Kött J, Hoehne IL, Heidrich I, Zimmermann N, Reese K-L, Zell T, Geidel G, Rünger A, Schneider SW, Pantel K, et al. High Serum Levels of CCL20 Are Associated with Recurrence and Unfavorable Overall Survival in Advanced Melanoma Patients Receiving Immunotherapy. Cancers. 2024; 16(9):1737. https://doi.org/10.3390/cancers16091737
Chicago/Turabian StyleKött, Julian, Inka Lilott Hoehne, Isabel Heidrich, Noah Zimmermann, Kim-Lea Reese, Tim Zell, Glenn Geidel, Alessandra Rünger, Stefan W. Schneider, Klaus Pantel, and et al. 2024. "High Serum Levels of CCL20 Are Associated with Recurrence and Unfavorable Overall Survival in Advanced Melanoma Patients Receiving Immunotherapy" Cancers 16, no. 9: 1737. https://doi.org/10.3390/cancers16091737