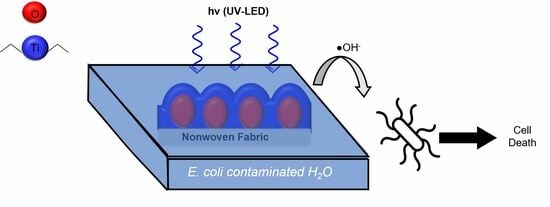
TiO2-Coated Meltblown Nonwoven Fabrics Prepared via Atomic Layer Deposition for the Inactivation of E. coli as a Model Photocatalytic Drinking Water Treatment System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Stock Preparation
2.2. Preparation of Meltblown Nonwoven Fabrics
2.3. Synthesis of TiO2 Films
2.4. Photocatalytic Studies
2.4.1. Ultraviolet Light-Emitting Diode Irradiation
2.4.2. Experimental Conditions
2.4.3. Inactivation Kinetics
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odonkor, S.T.; Ampofo, J.K. Escherichia coli as an Indicator of Bacteriological Quality of Water: An Overview. Microbiol. Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Vietro, N.D.; Tursi, A.; Beneduci, A.; Chidichimo, F.; Milella, A.; Fracassi, F.; Chatzisymeon, E.; Chidichimo, G. Photocatalytic Inactivation of Escherichia coli Bacteria in Water Using Low Pressure Plasma Deposited TiO2 Cellulose Fabric. Photochem. Photobiol. Sci. 2019, 18, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kettleson, E.; An, W.-J.; Tang, Y.J.; Biswas, P. Inactivation of E. coli in Water Using Photocatalytic, Nanostructured Films Synthesized by Aerosol Routes. Catalysts 2013, 3, 247–260. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Babalola, S.O.; Onwudiwe, D.C. Photocatalytic Inactivation as a Method of Elimination of E. coli from Drinking Water. Appl. Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Khani, M.; Amin, N.A.S.; Hosseini, S.N.; Heidarrezaei, M. Kinetics Study of the Photocatalytic Inactivation of Escherichia coli. IJNBM 2016, 6, 139. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Cruz, J.M.; Montagnolli, R.N.; Bidoia, E.D.; Lopes, P.R.M. Photocatalytic and Photoelectrochemical Inactivation of Escherichia coli and Staphylococcus aureus. Water Supply 2014, 15, 107–113. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Ciavola, M.; Rizzo, L.; McDowell, D.A.; Byrne, J.A. Effect of Photocatalysis on the Transfer of Antibiotic Resistance Genes in Urban Wastewater. Catal. Today 2015, 240, 55–60. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The Negative Impact of Antibiotic Resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic Inactivation of Escherischia Coli: Effect of Concentration of TiO2 and Microorganism, Nature, and Intensity of UV Irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Aragon, A.G.; Kierulf-Vieira, W.; Łęcki, T.; Zarębska, K.; Widera-Kalinowska, J.; Skompska, M. Synthesis and Application of N-Doped TiO2/CdS/Poly(1,8-Diaminocarbazole) Composite for Photocatalytic Degradation of 4-Chlorophenol under Visible Light. Electrochim. Acta 2019, 314, 73–80. [Google Scholar] [CrossRef]
- Badhe, R.A.; Ansari, A.; Garje, S.S. Study of Optical Properties of TiO2 Nanoparticles and CdS@TiO2 Nanocomposites and Their Use for Photocatalytic Degradation of Rhodamine B under Natural Light Irradiation. Bull. Mater. Sci. 2021, 44, 11. [Google Scholar] [CrossRef]
- Madkour, M.; Allam, O.G.; Abdel Nazeer, A.; Amin, M.O.; Al-Hetlani, E. CeO2-Based Nanoheterostructures with p–n and n–n Heterojunction Arrangements for Enhancing the Solar-Driven Photodegradation of Rhodamine 6G Dye. J. Mater. Sci. Mater. Electron. 2019, 30, 10857–10866. [Google Scholar] [CrossRef]
- He, Z.; Sun, C.; Yang, S.; Ding, Y.; He, H.; Wang, Z. Photocatalytic Degradation of Rhodamine B by Bi2WO6 with Electron Accepting Agent under Microwave Irradiation: Mechanism and Pathway. J. Hazard. Mater. 2009, 162, 1477–1486. [Google Scholar] [CrossRef]
- Olagunju, M.O.; Zahran, E.M.; Reed, J.M.; Zeynaloo, E.; Shukla, D.; Cohn, J.L.; Surnar, B.; Dhar, S.; Bachas, L.G.; Knecht, M.R. Halide Effects in BiVO4/BiOX Heterostructures Decorated with Pd Nanoparticles for Photocatalytic Degradation of Rhodamine B as a Model Organic Pollutant. ACS Appl. Nano Mater. 2021, 4, 3262–3272. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Chaoui, N.; Trzpit, M.; Ghazzal, N.; Robert, D.; Weber, J.V. UV–Vis versus Visible Degradation of Acid Orange II in a Coupled CdS/TiO2 Semiconductors Suspension. J. Photochem. Photobiol. A Chem. 2006, 183, 218–224. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 Films and Membranes for the Development of Efficient Wastewater Treatment and Reuse Systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Baghdadi, A.M.; Saddiq, A.A.; Aissa, A.; Algamal, Y.; Khalil, N.M. Structural Refinement and Antimicrobial Activity of Aluminum Oxide Nanoparticles. J. Ceram. Soc. Jpn. 2022, 130, 257–263. [Google Scholar] [CrossRef]
- Akyildiz, H.I.; Diler, S.; Islam, S. Evaluation of TiO2 and ZnO Atomic Layer Deposition Coated Polyamide 66 Fabrics for Photocatalytic Activity and Antibacterial Applications. J. Vac. Sci. Technol. A 2021, 39, 022405. [Google Scholar] [CrossRef]
- Pham, K.; Pelisset, S.; Kinnunen, N.; Karvinen, P.; Hakala, T.K.; Saarinen, J.J. Controlled Photocatalytic Activity of TiO2 Inverse Opal Structures with Atomic Layer Deposited (ALD) Metal Oxide Thin Films. Mater. Chem. Phys. 2022, 277, 125533. [Google Scholar] [CrossRef]
- Islam, S.; Akyildiz, H.I. Atomic Layer Deposition of TiO2 Thin Films on Glass Fibers for Enhanced Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2022, 33, 18002–18013. [Google Scholar] [CrossRef]
- Li, L.; Yu, P.; Li, Y.; Wu, X.; Li, W.; Cheng, X. A Facile Approach to Fabricating Antibacterial Textile with High Efficiency and Compact Process. Adv. Mater. Interfaces 2021, 8, 2101197. [Google Scholar] [CrossRef]
- Popescu, M.C.; Ungureanu, C.; Buse, E.; Nastase, F.; Tucureanu, V.; Suchea, M.; Draga, S.; Popescu, M.A. Antibacterial Efficiency of Cellulose-Based Fibers Covered with ZnO and Al2O3 by Atomic Layer Deposition. Appl. Surf. Sci. 2019, 481, 1287–1298. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Liu, Z.; Sang, L.; Yang, L.; Chen, Q. The Antibacterial Polyamide 6-ZnO Hierarchical Nanofibers Fabricated by Atomic Layer Deposition and Hydrothermal Growth. Nanoscale Res. Lett. 2017, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.; Kim, J.M.; Kim, M.K.; Kim, H.; Kim, W.H. Low-Temperature Atomic Layer Deposition of TiO2, Al2O3, and ZnO Thin Films. J. Korean Phys. Soc. 2011, 59, 452–457. [Google Scholar] [CrossRef]
- Aghaee, M.; Maydannik, P.S.; Johansson, P.; Kuusipalo, J.; Creatore, M.; Homola, T.; Cameron, D.C. Low Temperature Temporal and Spatial Atomic Layer Deposition of TiO2 Films. J. Vac. Sci. Technol. A 2015, 33, 041512. [Google Scholar] [CrossRef]
- Wu, P.; Imlay, J.A.; Shang, J.K. Mechanism of Escherichia coli Inactivation on Palladium-Modified Nitrogen-Doped Titanium Dioxide. Biomaterials 2010, 31, 7526–7533. [Google Scholar] [CrossRef]




| Fabric Type | Catalyst | External Light | Log Inactivation |
|---|---|---|---|
| PPW7 | TiO2 | UV-LED | 4.40 ± 0.043 |
| PPW8 | TiO2 | UV-LED | 4.40 ± 0.032 |
| PPW9 | TiO2 | UV-LED | 4.40 ± 0.041 |
| Laprotex™ | TiO2 | UV-LED | 5.00 ± 0.031 |
| - | - | UV-LED | 1.35 ± 0.0422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragon, A.G.; Cárdenas Sánchez, J.A.; Zimeri, C.; Shim, E.; Fang, X.; Young, K.R.L. TiO2-Coated Meltblown Nonwoven Fabrics Prepared via Atomic Layer Deposition for the Inactivation of E. coli as a Model Photocatalytic Drinking Water Treatment System. Environments 2024, 11, 92. https://doi.org/10.3390/environments11050092
Aragon AG, Cárdenas Sánchez JA, Zimeri C, Shim E, Fang X, Young KRL. TiO2-Coated Meltblown Nonwoven Fabrics Prepared via Atomic Layer Deposition for the Inactivation of E. coli as a Model Photocatalytic Drinking Water Treatment System. Environments. 2024; 11(5):92. https://doi.org/10.3390/environments11050092
Chicago/Turabian StyleAragon, Alexander G., Jaime A. Cárdenas Sánchez, Carlos Zimeri, Eunkyoung Shim, Xiaomeng Fang, and Kyana R. L. Young. 2024. "TiO2-Coated Meltblown Nonwoven Fabrics Prepared via Atomic Layer Deposition for the Inactivation of E. coli as a Model Photocatalytic Drinking Water Treatment System" Environments 11, no. 5: 92. https://doi.org/10.3390/environments11050092