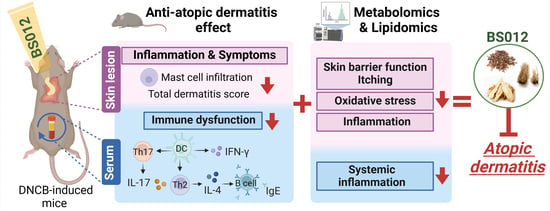
Exploring the Metabolic Effects of a Herbal Remedy of Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia Extracts: Unraveling Its Therapeutic Potential as a Topical Application for Atopic Dermatitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. BS012 Extract Preparation
2.2. Animal Housing
2.3. DNCB Induction of AD-Like Skin Lesions
2.4. Quantification of the Total Dermatitis Score
2.5. Assessment of Serum Immunoglobulin (IgE) and Inflammatory Cytokine Levels
2.6. Histological Analysis
2.7. Scratching Behavior
2.8. Sample Preparation and MS-Based Metabolomic and Lipidomic Profiling
3. Results
3.1. Effect of Topically Applied BS012 on DNCB-Induced Atopic Skin Lesions
3.2. Impact of BS012 on Serum Cytokines and Chemokine Levels in DNCB-Induced NC/Nga Mice
3.3. Changes in Serum Metabolites following Topical Application of BS012 in DNCB-Induced NC/Nga Mice
3.4. Changes in Skin Lesion Metabolites following the Topical Application of BS012 in DNCB-Induced NC/Nga Mice
3.5. Identification of Key Components of BS012 through the Profiling of Exogenous Metabolites in Skin Tissues following Topical Application
4. Discussion
4.1. The Topical Administration of BS012 Reduced Systemic Inflammation and Improved Metabolic Dysfunctions in the Serum of DNCB-Induced NC/Nga Mice
4.2. BS012 Topical Application Attenuates Immune Dysregulation and Oxidative Stress in DNCB-Induced NC/Nga Mouse Skin Lesions
4.3. Topical Application of BS012 Improved Skin Barrier Function and AD Symptoms in Damaged Skin Lesions of DNCB-Induced NC/Nga Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23 (Suppl. S8), S115–S123. [Google Scholar]
- Pavlis, J.; Yosipovitch, G. Management of Itch in Atopic Dermatitis. Am. J. Clin. Dermatol. 2018, 19, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Napolitano, M.; Patruno, C.; Villani, A.; Balato, A.; Monfrecola, G.; Ayala, F.; Balato, N. Systemic Treatment of Adult Atopic Dermatitis: A Review. Dermatol. Ther. 2017, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Gu, H.M.; Jeon, M.J.; Han, S.M.; Pak, S.C.; Lee, C.K.; Park, I.S.; et al. Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br. J. Pharmacol. 2018, 175, 4310–4324. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Hong, S.U.; Kim, H.T.; Seo, H.S.; Kim, K.; Ko, S.G.; Choi, I. A multicenter study on the efficacy and safety of So-Cheong-Ryong-Tang for perennial allergic rhinitis. Complement. Ther. Med. 2019, 45, 50–56. [Google Scholar] [CrossRef]
- Hyun Ok Yang, K.W.K.; Sung Won, K.; Jeong Hill, P.; Kim, H.; Oh, M.S.; Jang, D.S.; Lee, D.; Cheol, K.H.; Jung, B.H. Compositions for preventing or treating allergic disease comprising extracts of Asiasarum sieboldi, Platycodon grandiflorum and Cinnamomum cassia. South. Korea Patent No. 10-2262465, 28 May 2019. [Google Scholar]
- Lee, G.; Park, J.; Lee, H.; Kim, K.S.; Park, J.H.; Kyung, S.Y.; Kim, H.S.; Yang, H.O.; Jung, B.H. Anti-inflammatory effect and metabolic mechanism of BS012, a mixture of Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia extracts, on atopic dermatitis in vivo and in vitro. Phytomed. Int. J. Phytother. Phytopharm. 2023, 115, 154818. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Zhang, P.; Dai, X.; Gao, Y.; Lv, Y.; Qin, S.; Xu, F. Integrated Metabolomics and Network Pharmacology Strategy-Driven Active Traditional Chinese Medicine Ingredients Discovery for the Alleviation of Cisplatin Nephrotoxicity. Chem. Res. Toxicol. 2019, 32, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Takeuchi, H.; Sakuma, S.; Sengoku, T.; Takakura, S. Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Ski. Pharmacol. Physiol. 2009, 22, 240–247. [Google Scholar] [CrossRef]
- Lim, S.K.; Kwon, M.S.; Lee, J.; Oh, Y.J.; Jang, J.Y.; Lee, J.H.; Park, H.W.; Nam, Y.D.; Seo, M.J.; Roh, S.W.; et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci. Rep. 2017, 7, 40040. [Google Scholar] [CrossRef]
- Lee, G.; Park, Y.S.; Cho, C.; Lee, H.; Park, J.; Park, D.J.; Lee, J.H.; Lee, H.J.; Ha, T.K.; Kim, Y.J.; et al. Short-term changes in the serum metabolome after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Metabolomics Off. J. Metabolomic Soc. 2021, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 2013, 70, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.J.; Furlonger, C.; Williams, D.E.; Paige, C.J. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 1996, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Shiraishi, N.; Igeta, K.; Itoh, T.; Chikumoto, T.; Nagao, M.; Kim, J.F.; Nagai, H. Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur. J. Pharmacol. 2006, 546, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Folster-Holst, R.; Jensen, J.M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Alashmali, S.M.; Lin, L.; Trepanier, M.O.; Cisbani, G.; Bazinet, R.P. The effects of n-6 polyunsaturated fatty acid deprivation on the inflammatory gene response to lipopolysaccharide in the mouse hippocampus. J. Neuroinflamm. 2019, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, J.A.; Hofheinz, K.; Zaiss, M.M.; Kronke, G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Asahara, M.; Ito, N.; Hoshino, Y.; Sasaki, T.; Yokomizo, T.; Nakamura, M.; Shimizu, T.; Yamada, Y. Role of leukotriene B4 (LTB4)-LTB4 receptor 1 signaling in post-incisional nociceptive sensitization and local inflammation in mice. PLoS ONE 2022, 17, e0276135. [Google Scholar] [CrossRef]
- Blunder, S.; Ruhl, R.; Moosbrugger-Martinz, V.; Krimmel, C.; Geisler, A.; Zhu, H.; Crumrine, D.; Elias, P.M.; Gruber, R.; Schmuth, M.; et al. Alterations in Epidermal Eicosanoid Metabolism Contribute to Inflammation and Impaired Late Differentiation in FLG-Mutated Atopic Dermatitis. J. Investig. Dermatol. 2017, 137, 706–715. [Google Scholar] [CrossRef]
- Torocsik, D.; Weise, C.; Gericke, J.; Szegedi, A.; Lucas, R.; Mihaly, J.; Worm, M.; Ruhl, R. Transcriptomic and lipidomic profiling of eicosanoid/docosanoid signalling in affected and non-affected skin of human atopic dermatitis patients. Exp. Dermatol. 2019, 28, 177–189. [Google Scholar] [CrossRef]
- Nieman, D.C.; Meaney, M.P.; John, C.S.; Knagge, K.J.; Chen, H. 9- and 13-Hydroxy-octadecadienoic acids (9+13 HODE) are inversely related to granulocyte colony stimulating factor and IL-6 in runners after 2 h running. Brain Behav. Immun. 2016, 56, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Chung, M.J.; Seo, J.G. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4(+)Foxp3(+) T cells. Food Nutr. Res. 2016, 60, 32550. [Google Scholar] [CrossRef] [PubMed]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Ilves, L.; Ottas, A.; Kaldvee, B.; Abram, K.; Soomets, U.; Zilmer, M.; Jaks, V.; Kingo, K. Metabolomic Analysis of Skin Biopsies from Patients with Atopic Dermatitis Reveals Hallmarks of Inflammation, Disrupted Barrier Function and Oxidative Stress. Acta Derm.-Venereol. 2021, 101, adv00407. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Cao, S.; Yang, Z. Asparagine Is a Critical Limiting Metabolite for Vaccinia Virus Protein Synthesis during Glutamine Deprivation. J. Virol. 2019, 93, e01834-18. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131 (Suppl. S9), 2515S–2522S; discussion 2523S–2514S. [Google Scholar] [CrossRef] [PubMed]
- Bin, P.; Huang, R.; Zhou, X. Oxidation Resistance of the Sulfur Amino Acids: Methionine and Cysteine. BioMed Res. Int. 2017, 2017, 9584932. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wang, X.Q.; Oveisi, F.; Rad, B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 2000, 36, 142–146. [Google Scholar] [CrossRef]
- Nuhu, F.; Gordon, A.; Sturmey, R.; Seymour, A.M.; Bhandari, S. Measurement of Glutathione as a Tool for Oxidative Stress Studies by High Performance Liquid Chromatography. Molecules 2020, 25, 4196. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Nakamichi, N.; Nanmo, H.; Kimura, K.; Masuo, Y.; Sakai, Y.; Schinkel, A.H.; Sato, S.; Soga, T.; Kato, Y. Metabolome Analysis Reveals Dermal Histamine Accumulation in Murine Dermatitis Provoked by Genetic Deletion of P-Glycoprotein and Breast Cancer Resistance Protein. Pharm. Res. 2019, 36, 158. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the ceramide profile of atopic dermatitis patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef]
- Martin, G.E.; Boudreau, R.M.; Couch, C.; Becker, K.A.; Edwards, M.J.; Caldwell, C.C.; Gulbins, E.; Seitz, A. Sphingosine’s role in epithelial host defense: A natural antimicrobial and novel therapeutic. Biochimie 2017, 141, 91–96. [Google Scholar] [CrossRef]
- Sur, B.J.; Lee, B.; Yeom, M.; Han, J.J.; Choi, H.D.; Lee, H.; Kim, S.J.; Yoon, S.H.; Hahm, D.H. Inhibitory Effect of Phosphatidylserine on Atopy-like Dermatitis in NC/Nga Mice. Food Sci. Biotechnol. 2010, 19, 1513–1518. [Google Scholar] [CrossRef]
- Umehara, Y.; Kiatsurayanon, C.; Trujillo-Paez, J.V.; Chieosilapatham, P.; Peng, G.; Yue, H.; Nguyen, H.L.T.; Song, P.; Okumura, K.; Ogawa, H.; et al. Intractable Itch in Atopic Dermatitis: Causes and Treatments. Biomedicines 2021, 9, 229. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Ye, Y.M.; Kim, B.E.; Shin, Y.S.; Park, H.S.; Leung, D.Y.M. Increased epidermal filaggrin in chronic idiopathic urticaria is associated with severity of urticaria. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2014, 112, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, N.K.; Norval, M. Urocanic acid in the skin: A mixed blessing? J. Investig. Dermatol. 2011, 131, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.N.; Frade, M.A.C. Efficacy of 0.2% hyaluronic acid in the healing of skin abrasions in rats. Heliyon 2021, 7, e07572. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Jung, B.H.; Lee, T.; Park, J.H.; Kim, H.S.; Kim, H.; Yang, H.O. Exploring the Metabolic Effects of a Herbal Remedy of Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia Extracts: Unraveling Its Therapeutic Potential as a Topical Application for Atopic Dermatitis Treatment. Antioxidants 2024, 13, 563. https://doi.org/10.3390/antiox13050563
Lee G, Jung BH, Lee T, Park JH, Kim HS, Kim H, Yang HO. Exploring the Metabolic Effects of a Herbal Remedy of Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia Extracts: Unraveling Its Therapeutic Potential as a Topical Application for Atopic Dermatitis Treatment. Antioxidants. 2024; 13(5):563. https://doi.org/10.3390/antiox13050563
Chicago/Turabian StyleLee, Gakyung, Byung Hwa Jung, Taemin Lee, Jae Hyeon Park, Hyung Sik Kim, Hocheol Kim, and Hyun Ok Yang. 2024. "Exploring the Metabolic Effects of a Herbal Remedy of Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia Extracts: Unraveling Its Therapeutic Potential as a Topical Application for Atopic Dermatitis Treatment" Antioxidants 13, no. 5: 563. https://doi.org/10.3390/antiox13050563