Fluoroscopy-Guided Transgluteal Pudendal Nerve Block for Pudendal Neuralgia: A Retrospective Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Population
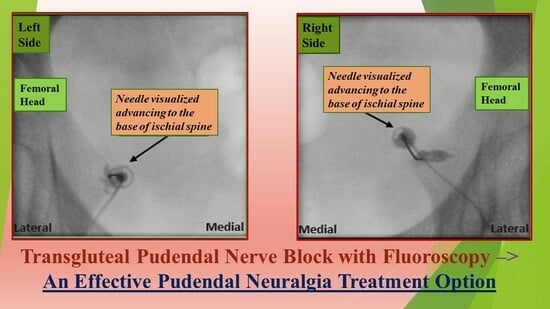
2.3. Pudendal Nerve Block
2.4. Outcome Measurements
3. Results
3.1. Participant Flow
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
4.1. Our Retrospective Case Series
4.2. Non-Interventional Treatment Options
4.2.1. Lifestyle Modifications
4.2.2. Physical Therapy
4.2.3. Cognitive Behavioral Therapy
4.2.4. Pharmacological Therapy
4.3. Interventional Treatment Options
4.3.1. Nerve Block
4.3.2. Radiofrequency Ablation
4.3.3. Neuromodulation
4.3.4. Surgery
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hibner, M.; Desai, N.; Robertson, L.J.; Nour, M. Pudendal neuralgia. J. Minim. Invasive Gynecol. 2010, 17, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, G.; Lanoe, Y.; Perrigot, M.; Goudal, H. A new canal syndrome: Compression of the pudendal nerve in Alcock’s canal or perinal paralysis of cyclists. Presse Med. 1987, 16, 399. [Google Scholar] [PubMed]
- Labat, J.J.; Riant, T.; Robert, R.; Amarenco, G.; Lefaucheur, J.P.; Rigaud, J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol. Urodyn. 2008, 27, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Leslie, S.W.; Singh, P. Pudendal Nerve Entrapment Syndrome; 21 August 2023; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Choi, S.S.; Lee, P.B.; Kim, Y.C.; Kim, H.J.; Lee, S.C. C-arm-guided pudendal nerve block: A new technique. Int. J. Clin. Pract. 2006, 60, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Pritchett, Y.L.; Robinson, M.; Prakash, A.; Chappell, A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: Analyses of data from clinical trials of duloxetine in pain disorders. J. Pain 2010, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Antolak, S.; Feloney, M.P.; Soon-Sutton, T.L. Pudendal Neuralgia; 28 November 2022; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Khoder, W.; Hale, D. Pudendal neuralgia. Obstet. Gynecol. Clin. North Am. 2014, 41, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Gupta, M.; Shankar, N.; Jain, S.; Saxena, A. Effects of yogic intervention on pain scores and quality of life in females with chronic pelvic pain. Int. J. Yoga 2017, 10, 9–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levesque, A.; Bautrant, E.; Quistrebert, V.; Valancogne, G.; Riant, T.; Beer Gabel, M.; Leroi, A.M.; Jottard, K.; Bruyninx, L.; Amarenco, G.; et al. Recommendations on the management of pudendal nerve entrapment syndrome: A formalised expert consensus. Eur. J. Pain 2022, 26, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Vallinga, M.S.; Spoelstra, S.K.; Hemel, I.L.; van de Wiel, H.B.; Weijmar Schultz, W.C. Transcutaneous electrical nerve stimulation as an additional treatment for women suffering from therapy-resistant provoked vestibulodynia: A feasibility study. J. Sex. Med. 2015, 12, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, B. Physiotherapy for pelvic pain and female sexual dysfunction: An untapped resource. Int. Urogynecol J. 2018, 29, 631–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehde, D.M.; Dillworth, T.M.; Turner, J.A. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am. Psychol. 2014, 69, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Callan, J.; Moore, W.C.; Fuller, M.C.; Renschler, J.S.; Fisher, P.; Jung, J.W.; Hasoon, J.; Eskander, J.; Kaye, A.D.; et al. Cognitive behavioral therapy for the treatment of chronic pelvic pain. Best. Pract. Res. Clin. Anaesthesiol. 2020, 34, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Parkitny, L.; McLain, D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin. Rheumatol. 2014, 33, 451–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKenzie-Brown, A.M.; Boorman, D.W.; Ibanez, K.R.; Agwu, E.; Singh, V. Low-Dose Naltrexone (LDN) for Chronic Pain at a Single Institution: A Case Series. J. Pain Res. 2023, 16, 1993–1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastler, A.; Puget, J.; Tiberghien, F.; Pellat, J.M.; Krainik, A.; Kastler, B. Dual Site Pudendal Nerve Infiltration: More than Just a Diagnostic Test? Pain Physician 2018, 21, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, G.A.; Bhatia, A.; Chan, C.W.; Peng, P.W. Randomized controlled trial comparing pudendal nerve block under ultrasound and fluoroscopic guidance. Reg. Anesth. Pain Med. 2012, 37, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Lee, Y.J.; Kim, J.Y.; Lee, W.Y.; Lim, Y.H. Treatment of radiation-induced vulvar pain via pudendal nerve block under fluoroscopic guidance. Urol. Case Rep. 2020, 33, 101282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.; Lee, C.J.; Lee, J.Y.; Kim, T.H.; Sim, W.S.; Lee, S.Y.; Hwang, H.Y. Fluoroscopy-guided pudendal nerve block and pulsed radiofrequency treatment: A case report. Korean J. Anesthesiol. 2009, 56, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Collard, M.D.; Xi, Y.; Patel, A.A.; Scott, K.M.; Jones, S.; Chhabra, A. Initial experience of CT-guided pulsed radiofrequency ablation of the pudendal nerve for chronic recalcitrant pelvic pain. Clin. Radiol. 2019, 74, 897.e17–897.e23. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, J.; Yang, Y.; Ye, L.; Wang, X. Clinical effect and safety of pulsed radiofrequency treatment for pudendal neuralgia: A prospective, randomized controlled clinical trial. J. Pain. Res. 2018, 11, 2367–2374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, K.M.; Killinger, K.A.; Jaeger, C.; Chen, C. Pilot Study Exploring Chronic Pudendal Neuromodulation as a Treatment Option for Pain Associated with Pudendal Neuralgia. Low. Urin. Tract. Symptoms. 2015, 7, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Buffenoir, K.; Rioult, B.; Hamel, O.; Labat, J.J.; Riant, T.; Robert, R. Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: A prospective study of 27 consecutive cases. Neurourol. Urodyn. 2015, 34, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.W.; Yang, A. Dorsal Root Ganglion Stimulation for Chronic Pelvic Pain: A Case Series and Technical Report on a Novel Lead Configuration. Neuromodulation 2019, 22, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Gulve, A.; ElDabe, S.; Baranidharan, G.; Wolf, K.; Demmel, W.; Rasche, D.; Sharma, M.; Klase, D.; Jahnichen, G.; et al. Spinal cord stimulation of the dorsal root ganglion for groin pain-a retrospective review. Pain Pract. 2015, 15, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.; Paszkiewicz, E.; Kirkpatrick, C.; Hinkel, B.; Oleson, K. Sacral nerve stimulation in patients with chronic intractable pelvic pain. J. Urol. 2001, 166, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, J.; Naldini, G.; Carriero, A. Sacral nerve modulation in the treatment of chronic pelvic pain. Int. J. Colorectal Dis. 2012, 27, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Sokal, P.; Zieliński, P.; Harat, M. Sacral roots stimulation in chronic pelvic pain. Neurol. Neurochir. Pol. 2015, 49, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Vancaillie, T.; Kite, L.; Howard, E.; Chow, J. Sacral neuromodulation for pelvic pain and pelvic organ dysfunction: A case series. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Waxweiler, C.; Dobos, S.; Thill, V.; Bruyninx, L. Selection criteria for surgical treatment of pudendal neuralgia. Neurourol. Urodyn. 2017, 36, 663–666. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | Mean ± Standard Deviation |
|---|---|
| Age (years) | 43.6 ± 16.1 |
| BMI (kg/m2) | 29.2 ± 12.9 |
| Gender (Female) | 63 (77.8) |
| Gender (Male) | 18 (22.2) |
| Past medical history | N (%) |
| Anxiety | 29 (35.8) |
| Depression | 23 (28.4) |
| Autoimmune conditions | 28 (34.6) |
| Inflammatory bowel disease | 11 (13.6) |
| Gynecological/urological surgery | 49 (60.4) |
| Traumatic birth | 5 (5.2) |
| Prior Treatments | N (%) |
| Lifestyle modifications | 18 (22.2) |
| Psychotherapy | 1 (1.2) |
| Acupuncture | 8 (9.9) |
| Pelvic floor physical therapy | 57 (70.4) |
| Pharmacological medications | 74 (91.4) |
| Other procedures/surgeries | 49 (60.5) |
| Complications | N (%) |
|---|---|
| Fecal incontinence on day of procedure | 1 (1.2) |
| Leg weakness/numbness | 7 (8.6) |
| Pelvic floor spasm | 1 (1.2) |
| Pain flare-up | 6 (7.4) |
| Back pain | 1 (1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levin, D.; Van Florcke, D.; Schmitt, M.; Kendall, L.K.; Patel, A.; Doan, L.V.; Kirpekar, M. Fluoroscopy-Guided Transgluteal Pudendal Nerve Block for Pudendal Neuralgia: A Retrospective Case Series. J. Clin. Med. 2024, 13, 2636. https://doi.org/10.3390/jcm13092636
Levin D, Van Florcke D, Schmitt M, Kendall LK, Patel A, Doan LV, Kirpekar M. Fluoroscopy-Guided Transgluteal Pudendal Nerve Block for Pudendal Neuralgia: A Retrospective Case Series. Journal of Clinical Medicine. 2024; 13(9):2636. https://doi.org/10.3390/jcm13092636
Chicago/Turabian StyleLevin, Danielle, Daniel Van Florcke, Monika Schmitt, Lucinda Kurzava Kendall, Alopi Patel, Lisa V. Doan, and Meera Kirpekar. 2024. "Fluoroscopy-Guided Transgluteal Pudendal Nerve Block for Pudendal Neuralgia: A Retrospective Case Series" Journal of Clinical Medicine 13, no. 9: 2636. https://doi.org/10.3390/jcm13092636