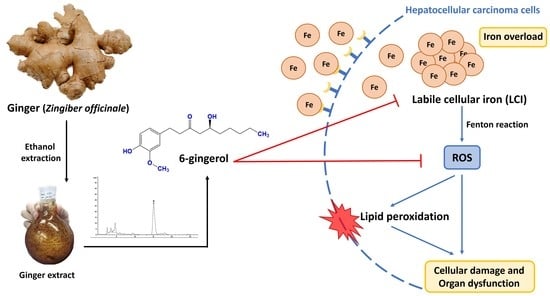
Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells
Abstract
:1. Introduction
2. Results
2.1. Chemical Compositions of Ginger Extract
2.2. Antioxidant Activity of the Ginger Extract
2.3. Antihemolytic Activity of the Ginger Extract
2.4. The Effect of the Ginger Extract on Cell Viability
2.5. Intracellular Iron Chelating Activity of the Ginger Extract
2.6. The Intracellular Free-Radical Scavenging Activity of the Ginger Extract
2.7. Inhibitory Effect of the Ginger Extract on Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Chemical Reagent
4.2. Plant Extraction
4.3. Chemical Composition Determination
4.3.1. Total Phenolic Content
4.3.2. Determination of 6-Gingerol
4.4. Antioxidant Activity Measurement
4.4.1. DPPH Assay
4.4.2. ABTS Assay
4.4.3. AAPH Assay
4.5. Cell Culture
4.6. Cytotoxicity Assay
4.7. Determination of Oxidative Stress Markers
4.7.1. Intracellular ROS
4.7.2. Lipid Peroxidation
4.8. Determination of Labile Cellular Iron
4.9. Ethical Approval
4.10. Statistic
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antioxid. Redox Sig-Naling 2014, 20, 1917–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.; Faustino, P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Ward, R. An update on disordered iron metabolism and iron overload. Hematology 2010, 15, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. Int. Symp. Home Hemodial. 2017, 21 (Suppl. S1), S6–S20. [Google Scholar] [CrossRef] [Green Version]
- Ramm, G.A.; Ruddell, R.G. Hepatotoxicity of Iron Overload: Mechanisms of Iron-Induced Hepatic Fibrogenesis. Semin. Liver Dis. 2005, 25, 433–449. [Google Scholar] [CrossRef]
- Ganne-Carrié, N.; Christidis, C.; Chastang, C.; Ziol, M.; Chapel, F.; Imbert-Bismut, F.; Trinchet, J.-C.; Guettier, C.; Beaugrand, M. Liver iron is predictive of death in alcoholic cirrhosis: A multivariate study of 229 consecutive patients with alcoholic and/or hepatitis C virus cirrhosis: A prospective follow up study. Gut 2000, 46, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Shah, R.; Muriel, P. Oxidative Stress and Inflammation in Hepatic Diseases: Current and Future Therapy. Oxidative Med. Cell. Longev. 2017, 2017, 3140673. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic ap-plications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [Green Version]
- Godwill, A.E. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. In Phytochemicals; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 4. [Google Scholar]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crops Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological Properties of 6-Gingerol: A Brief Review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Tyagi, A.K. Ginger and Its Constituents: Role in Prevention and Treatment of Gastrointestinal Cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [Green Version]
- Asamenew, G.; Kim, H.-W.; Lee, M.-K.; Lee, S.-H.; Kim, Y.J.; Cha, Y.-S.; Yoo, S.M.; Kim, J.-B. Characterization of phenolic compounds from normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC–DAD–QToF–MS. Eur. Food Res. Technol. 2019, 245, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.J.; Frazer, D.M. Hepatic Iron Metabolism. Semin. Liver Dis. 2005, 25, 420–432. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Yu, X.; Ding, H.; Han, J.; Feng, J. Effects of intracellular iron overload on cell death and identification of potent cell death inhibitors. Biochem. Biophys. Res. Commun. 2018, 503, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Elshafey, M.M. Cross-talk between PPARγ, NF-κB, and p38 MAPK signaling mediates the ameliorating effects of bergenin against the iron overload-induced hepatotoxicity. Chem. Biol. Interact. 2022, 368, 110207. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and an-ti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Kabel, A.M. Taxifolin ameliorates iron overload-induced hepatocellular injury: Modulating PI3K/AKT and p38 MAPK signaling, inflammatory response, and hepatocellular regeneration. Chem. Biol. Interact. 2020, 330, 109230. [Google Scholar] [CrossRef]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharmacother. 2017, 87, 443–450. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiong, X.; Zhou, Y.; Wu, F.; Shao, Q.; Dong, R.; Liu, Q.; Li, L.; Chen, G. 6-gingerol ameliorates metabolic disorders by inhibiting hypertrophy and hyperplasia of adipocytes in high-fat-diet induced obese mice. Biomed. Pharmacother. 2022, 146, 112491. [Google Scholar] [CrossRef]
- Manjunathan, T.; Guru, A.; Arokiaraj, J.; Gopinath, P. 6-Gingerol and Semisynthetic 6-Gingerdione Counteract Oxidative Stress Induced by ROS in Zebrafish. Chem. Biodivers. 2021, 18, e2100650. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, J.; Wu, G.; Hu, Z.; Ying, P.; Bao, Z.; Ding, Z.; Tan, X. 6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway. Oxid. Med. Cell Longev. 2022, 2022, 3027514. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Paradee, N.; Howes, M.J.R.; Utama-ang, N.; Chaikitwattna, A.; Hider, R.C.; Srichairatanakool, S. A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human he-patoma (HuH7) cells. Phytother. Res. 2019, 33, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.-J.; Jeong, W.-S.; Kim, K.-B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-F.; Shen, H.-M.; Ong, C.-N. Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem. Pharmacol. 1999, 57, 273–279. [Google Scholar] [CrossRef] [PubMed]








| Extract/Control | TPC (mg GAE/g Extract) | 6-Gingerol (µg/mL) | ABTS IC50 (mg/mL) | DPPH IC50 (mg/mL) |
|---|---|---|---|---|
| Ginger extract | 43.32 ± 1.60 | 219.7 ± 9.69 | 1.43 ± 0.06 | 0.064 ± 0.002 |
| Trolox | - | - | 0.55 ± 0.07 | 0.012 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuljerm, H.; Paradee, N.; Katekaew, D.; Nantachai, P.; Settakorn, K.; Srichairatanakool, S.; Koonyosying, P. Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells. Plants 2023, 12, 2936. https://doi.org/10.3390/plants12162936
Chuljerm H, Paradee N, Katekaew D, Nantachai P, Settakorn K, Srichairatanakool S, Koonyosying P. Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells. Plants. 2023; 12(16):2936. https://doi.org/10.3390/plants12162936
Chicago/Turabian StyleChuljerm, Hataichanok, Narisara Paradee, Dabudsawin Katekaew, Panaphat Nantachai, Kornvipa Settakorn, Somdet Srichairatanakool, and Pimpisid Koonyosying. 2023. "Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells" Plants 12, no. 16: 2936. https://doi.org/10.3390/plants12162936