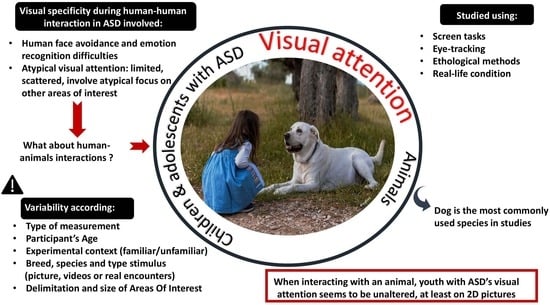
How Do Children and Adolescents with ASD Look at Animals? A Scoping Review
Abstract
:1. Introduction
1.1. Definitions
1.2. Characteristics of Vision in Individuals with ASD
1.3. Understanding Human Partners: Perception of Social Stimuli
1.4. Understanding a Non-Human Partner
1.5. Perception: Mechanisms Contributing to Differences between Humans and Non-Humans
2. Materials and Methods
2.1. Search Strategy
2.2. Search Protocol
2.3. Eligibility Criteria
2.4. Charting Data
3. Results
3.1. Study Selection
3.2. Characteristics of Publications
3.3. Methodological Aspects
| Authors | Visual Acuity Check | Type of Attention Measurement | Details of the Type of Measure of Attention to Animals | Duration of Attention Measurements (min; s) | Scenario Conditions and Place of Evaluation |
|---|---|---|---|---|---|
| New et al. [89] | NA | Responses to a computerized test: mouse click responses | Performance in change detection (speed and accuracy): index of automatic attentional prioritization for each type of object that changes between two pictures. The changing target in each scene was either an animate object (person or animal) or an inanimate object (plant or artifact) either with reversed orientation or that repeatedly disappeared and reappeared. | 14 images containing animals’ pictures out of a total of 56 images; 0.5 s for the first apparition of the image and until 20 s for the detection | Laboratory (room) |
| McPartland et al. [90] | Presence of eyeglasses as an exclusion criterion (n = 1) | Eye-tracking | Proportion of total fixation duration spent on each AOI (i.e., upper and lower areas of stimuli: mouth and eye position). AOI sizes and positions were equivalent across stimulus categories. 240 Hz. | 10 animal stimuli out of a total of 50 stimuli, 8 s of apparition | Laboratory (room) |
| Grandgeorge et al. [91] | NA | Ethological methods | Looking at and glancing at one of the target items (i.e., parent, observer, animal, or environment). Binary variables (presence/absence). | 15 min of exposure to an animal | Naturalist (at home) |
| O’Haire et al. [92] | NA | Direct observation (coding system = The Observation of Human-Animal Interaction for Research) | Gazing at (either a human animal or toy). Binary variables (presence/absence) during each 10-s interval over minutes pre-selected for coding. | Three sessions of 10 min exposure to an animal. Measurement of the first 10 min, including 3 min selected for coding | Naturalist (outside the school classroom) |
| Guillon et al. [93] | Yes | Eye-tracking | Number of first fixations on the left or right visual hemifield on stimuli (i.e., faces, objects). Number of non-lateralized fixations. A laterality index of the first fixation was used. 60 Hz. | 12 animal stimuli out of 28; 3.5 s of apparition | Laboratory (room) |
| Grandgeorge et al. [94] | NA | Ethological methods | Gaze (frequency in %) directed at: guinea pig, human observer, parent, objects—either unfamiliar or familiar—or self-centered. | 15 min of exposure to an animal. One exposure was stopped after 12 min | Naturalist (at home) |
| Muszkat et al. [95] | NA | Eye-tracking | Total number of fixations and total fixation time on two AOIs of equal size on facial stimuli (i.e., eye and mouth of either a dog or a human face). 300 Hz. | NA number of animal pictures, 5 s of apparition | Laboratory (room) |
| Grandgeorge et al. [80] | Vision acuity was an exclusion criterion: it had to be normal or corrected-to-normal vision (checked using the Monoyer’s scale of visual acuity). | Eye-tracking | Mean duration of fixation on three AOIs on each face (i.e., eyes, mouth, and ears for animals). The data were adjusted for variance in AOI size between stimuli. 60 Hz. | Six pictures of each animal, 5 s of apparition | Laboratory (room) |
| Grandgeorge et al. [96] | NA | Ethological methods | Gazes and glances (<1 s) occurrence and duration (s) based on eye/head orientation toward different targets (i.e., service dog, animal trainer, service dog and animal trainer dyad, environment including objects). Occurrences of joint attention were also collected. | S1: 30 min S2: 20 min of exposure to animals | Naturalist/Semi-standardization (room) |
| Doherty et al. [97] | NA | Response on touch screen | Accuracy and total time to complete the task. For each trial, participants had to touch the same type of target (animals) and avoid distractors (inanimate objects). three additional measures were produced: (1) Q score (2) best R (3) intersections rate (Score Q: “a measure of search efficiency combining speed and accuracy”, Best R: “a measure of horizontal or vertical spatial organization”; Intersections rate: “number of times the search path crosses over itself, divided by the amount of cancellations that are not immediate revisits” [109].) | No time limit for a trial; the trial ended when children touched 18 of the 20 targets (i.e., cats or animals or dogs) or emitted 40 responses, including some of the 70 distractors | Laboratory |
| Strathearn et al. [98] | Visual impairment was an exclusion criterion | Eye-tracking | Total duration and number of fixations on four AOIs on four pictures with different levels of organization (i.e., least systemized, less systemized, more systemized, most systemized). 60 Hz. | NA animal stimuli, 12 s of apparition | Laboratory |
| Germone et al. [99] | NA | Direct observation (coding system = The Observation of Human-Animal Interaction for Research) | Timed interval behavior coding system designed to quantify social communication and interactions with animals and control objects, including animal-directed gaze. | 10 min with an animal, including 3 min selected for coding | Laboratory (open classroom) |
| Uccheddu et al. [83] | NA | Parent questionnaire | Including a question about the child’s attention towards dogs: “Was the child able to pay more attention to dogs in daily routine?” Answers: Yes/No. | 30 min of exposure to an animal | Laboratory (room) |
| Gale et al. [100] | NA | Response on touch screen | Frequency of touch on each image (i.e., indicate preference) for each session. Pixelated movies of non-social stimuli (six different movies of abstract geometric moving patterns) and social stimuli (six different movies of dogs’ faces). | Six animal stimuli; if an animal was selected, it appeared for 1 min 30 | Laboratory (child’s home, nursery, or clinic setting) |
| Yamashiro et al. [101] | NA | Eye-tracking | Total length of fixations in each oval AOI corresponding to a face or non-face stimuli (i.e., two human faces, two monkey faces, and their corresponding non-face stimuli presented simultaneously). 60–120 Hz. | Two pictures of an animal. Each trial lasted until the infant accumulated 10 s of looking time at either display or for a maximum of 20 s | Laboratory (room) |
| Grandgeorge et al. [102] | NA | Parent questionnaires and ethological methods. | Parent-based short questionnaire: frequency of visual interaction between their child and their pet (never, rarely, often). In addition, observation at home: glance, mutual gaze, gaze towards the animal. | 60 min session of observation | Naturalist (at home) |
| Avila-Alvarez et al. [103] | NA | Questionnaire for health professionals | Brief questionnaire (Animal-assisted Therapy Flow Sheet). A scale was applied through direct observation of three sessions by an experienced health professional. It included frequency of «Looked at dog» (never, once, two or three times, and several times). | One session with an animal for around 20 min. Measurements were performed on one of the three sessions the child attended | Naturalist/Semi-standardized approach (room) |
| Valiyamattam et al. [84] | Yes | Eye-tracking | Gaze fixation durations on six AOIs on each human and animal face (i.e., face, left eye, right eye, eye region, mouth, and screen). Face AOIs were defined using the following landmarks: human hairline, ears, and nose tips. 120 Hz. | 20 pictures of animals, 5 s of apparition | Laboratory (room) |
| Dollion et al. [82] | Yes | Eye-tracking and Ethological methods | Number and duration of fixations in six AOIs on the visual scene: the whole service dog, the service dog’s head, the evaluator’s head, the parent’s head, the board games, the service dog’s accessories, and the rest of the visual scene. Each AOI was defined to encompass the entire element of interest (+additional space around the target). Additionally, based on observation, occurrences and durations of gazes directed towards: parent, service dog, board games, service, dog’s accessories, other directions. Eye-tracking glasses at 60 Hz. | S1: 20 min of exposure and measurement S2: eye-tracking measurement: 4 min; observation: 20 min of exposure and measurement | Laboratory (room) |
| Scheerer et al. [104] | Yes | S1: Response on the keyboard S2: Response on the keyboard and eye-tracking | S1: accuracy and response time in an attentional capture paradigm (i.e., detect whether a target butterfly was present or absent among distractors (face, train, fruit, objects, etc.)). S2: total number of fixations and latency of first fixation to an AOI (i.e., butterfly, neutral stimuli). AOIs were defined as a 3 cm by 3 cm region around the stimuli. 1000 Hz. | S1 and S2: one trial: 0 to 2 pictures of animals among six stimuli. 120 trials total, with an apparition until the subject’s response or 5 s maximum | Laboratory (room) |
| Dollion et al. [105] | NA | Ethological methods | Gaze (frequency in %) directed at: service dog parent games and objects other. | 20 min of exposure to an animal | Laboratory (room) |
3.4. Main Outcomes Results about Visual Attention
3.4.1. Attentional Engagement and Detection
3.4.2. Visual Exploration: Visual Preference for Humans versus Animals versus Others, i.e., What Do Children and Adolescents Look at?
3.4.3. Visual Exploration: Visual Exploration of Animal Faces
3.4.4. Visual Exploration: Visual Exploration in Relation to Species
3.4.5. Behavior
3.5. Limits
4. Discussion
4.1. Methodological Variations: Implications and Contribution to Our Current Knowledge
4.2. Implications of Visual Attention on the Benefits of Human-Animal Interactions
4.3. Variation Factors
4.4. Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Search Equation
References
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Arlington VA, USA, 2013; ISBN 978-0-89042-557-2. [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992; Volume 1. [Google Scholar]
- Simmons, D.R.; Robertson, A.E.; McKay, L.S.; Toal, E.; McAleer, P.; Pollick, F.E. Vision in Autism Spectrum Disorders. Vis. Res. 2009, 49, 2705–2739. [Google Scholar] [CrossRef]
- Hill, D. Tell Me No Lies: Using Science to Connect with Consumers. J. Interact. Mark. 2003, 17, 61–72. [Google Scholar] [CrossRef]
- Evans, K.K.; Horowitz, T.S.; Howe, P.; Pedersini, R.; Reijnen, E.; Pinto, Y.; Kuzmova, Y.; Wolfe, J.M. Visual Attention. WIREs Cogn. Sci. 2011, 2, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.M.; Wolfe, J.M. Visual Attention. In Blackwell Handbook of Sensation and Perception; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 272–310. ISBN 978-0-470-75347-7. [Google Scholar]
- Chokron, S.; Pieron, M.; Zalla, T. Troubles du spectre de l’autisme et troubles de la fonction visuelle: Revue critique, implications théoriques et cliniques. Inf. Psychiatr. 2014, 90, 819–826. [Google Scholar] [CrossRef]
- Cozolino, L. The Neuroscience of Human Relationships: Attachment and the Developing Social Brain, 2nd ed.; Norton Series on Interpersonal Neurobiology; W. W. Norton & Company: New York, NY, USA, 2014; ISBN 978-0-393-70782-3. [Google Scholar]
- Chokron, S.; Cavézian, C.; de Agostini, M. Troubles neurovisuels chez l’enfant: Sémiologie, retentissement sur les apprentissages et dépistage. Développements 2010, 6, 17–25. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Stuart, G.W.; Falkmer, M.; Ordqvist, A.; Leung, D.; Foster, J.K.; Falkmer, T. Brief Report: Visual Acuity in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2014, 44, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Griffiths, H.; Buckley, D.; Scope, A. Vision in Children and Adolescents with Autistic Spectrum Disorder: Evidence for Reduced Convergence. J. Autism Dev. Disord. 2009, 39, 965–975. [Google Scholar] [CrossRef]
- Bölte, S.; Schlitt, S.; Gapp, V.; Hainz, D.; Schirman, S.; Poustka, F.; Weber, B.; Freitag, C.; Ciaramidaro, A.; Walter, H. A Close Eye on the Eagle-Eyed Visual Acuity Hypothesis of Autism. J. Autism Dev. Disord. 2012, 42, 726–733. [Google Scholar] [CrossRef]
- Falkmer, M.; Stuart, G.W.; Danielsson, H.; Bram, S.; Lönebrink, M.; Falkmer, T. Visual Acuity in Adults with Asperger’s Syndrome: No Evidence for “Eagle-Eyed” Vision. Biol. Psychiatry 2011, 70, 812–816. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Masataka, N. Atypical Color Preference in Children with Autism Spectrum Disorder. Front. Psychol. 2016, 7, 1976. [Google Scholar] [CrossRef]
- Zachi, E.C.; Costa, T.L.; Barboni, M.T.S.; Costa, M.F.; Bonci, D.M.O.; Ventura, D.F. Color Vision Losses in Autism Spectrum Disorders. Front. Psychol. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Franklin, A.; Sowden, P.; Burley, R.; Notman, L.; Alder, E. Color Perception in Children with Autism. J. Autism Dev. Disord. 2008, 38, 1837–1847. [Google Scholar] [CrossRef]
- Happé, F. The Weak Central Coherence Account of Autism. In Handbook of Autism and Pervasive Developmental Disorders; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 640–649. ISBN 978-0-470-93934-5. [Google Scholar]
- Navon, D. Forest before Trees: The Precedence of Global Features in Visual Perception. Cognit. Psychol. 1977, 9, 353–383. [Google Scholar] [CrossRef]
- Happé, F.; Frith, U. The Weak Coherence Account: Detail-Focused Cognitive Style in Autism Spectrum Disorders. J. Autism Dev. Disord. 2006, 36, 5–25. [Google Scholar] [CrossRef]
- Posner, M.I. Orienting of Attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- Robertson, C.E.; Kravitz, D.J.; Freyberg, J.; Baron-Cohen, S.; Baker, C.I. Tunnel Vision: Sharper Gradient of Spatial Attention in Autism. J. Neurosci. 2013, 33, 6776–6781. [Google Scholar] [CrossRef]
- McCleery, J.P.; Allman, E.; Carver, L.J.; Dobkins, K.R. Abnormal Magnocellular Pathway Visual Processing in Infants at Risk for Autism. Biol. Psychiatry 2007, 62, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Zammarchi, G.; Conversano, C. Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis. Vision 2021, 5, 56. [Google Scholar] [CrossRef]
- Kovarski, K.; Meaux, E.; Batty, M. De l’exploration à la perception des visages chez les personnes avec un trouble du spectre de l’autisme. In Neuropsychologie et Remédiations des Troubles du Spectre de L’autisme; Neuropsychologie; De Boeck Supérieur: Louvain-la-Neuve, Belgium, 2018; Chapitre 5; pp. 137–187. ISBN 978-2-35327-433-8. [Google Scholar]
- Harrop, C.; Jones, D.; Zheng, S.; Nowell, S.; Schultz, R.; Parish-Morris, J. Visual Attention to Faces in Children with Autism Spectrum Disorder: Are There Sex Differences? Mol. Autism 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Harwood, R.; Kasari, C. The Art of Camouflage: Gender Differences in the Social Behaviors of Girls and Boys with Autism Spectrum Disorder. Autism 2017, 21, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Parish-Morris, J.; Liberman, M.Y.; Cieri, C.; Herrington, J.D.; Yerys, B.E.; Bateman, L.; Donaher, J.; Ferguson, E.; Pandey, J.; Schultz, R.T. Linguistic Camouflage in Girls with Autism Spectrum Disorder. Mol. Autism 2017, 8, 48. [Google Scholar] [CrossRef]
- Sacrey, L.-A.R.; Bryson, S.E.; Zwaigenbaum, L. Prospective Examination of Visual Attention during Play in Infants at High-Risk for Autism Spectrum Disorder: A Longitudinal Study from 6 to 36 Months of Age. Behav. Brain Res. 2013, 256, 441–450. [Google Scholar] [CrossRef]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The Impact of Atypical Sensory Processing on Social Impairments in Autism Spectrum Disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef]
- Klin, A.; Jones, W.; Schultz, R.; Volkmar, F.; Cohen, D. Visual Fixation Patterns during Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals with Autism. Arch. Gen. Psychiatry 2002, 59, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Teunisse, J.-P.; Gelder, B.D. Do Autistics Have a Generalized Face Processing Deficit? Int. J. Neurosci. 1994, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klin, A.; Sparrow, S.S.; de Bildt, A.; Cicchetti, D.V.; Cohen, D.J.; Volkmar, F.R. A Normed Study of Face Recognition in Autism and Related Disorders. J. Autism Dev. Disord. 1999, 29, 499–508. [Google Scholar] [CrossRef]
- Dawson, G.; Osterling, J.; Meltzoff, A.N.; Kuhl, P. Case Study of the Development of an Infant with Autism from Birth to Two Years of Age. J. Appl. Dev. Psychol. 2000, 21, 299–313. [Google Scholar] [CrossRef]
- Grandin, T. How People with Autism Think. In Learning and Cognition in Autism; Schopler, E., Mesibov, G.B., Eds.; Current Issues in Autism; Springer: Boston, MA, USA, 1995; pp. 137–156. ISBN 978-1-4899-1286-2. [Google Scholar]
- Suzanne Scherf, K.; Behrmann, M.; Minshew, N.; Luna, B. Atypical Development of Face and Greeble Recognition in Autism. J. Child Psychol. Psychiatry 2008, 49, 838–847. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Mottron, L.; Burack, J.A. Sensory, Motor and Attention Characteristics of Autistic Children. 2012. Available online: http://www.child-encyclopedia.com/autism/according-experts/sensory-motor-and-attention-characteristics-autistic-children (accessed on 3 February 2024).
- Gross, T.F. The Perception of Four Basic Emotions in Human and Nonhuman Faces by Children with Autism and Other Developmental Disabilities. J. Abnorm. Child Psychol. 2004, 32, 469–480. [Google Scholar] [CrossRef]
- Nagy, E.; Prentice, L.; Wakeling, T. Atypical Facial Emotion Recognition in Children with Autism Spectrum Disorders: Exploratory Analysis on the Role of Task Demands. Perception 2021, 50, 819–833. [Google Scholar] [CrossRef]
- Law Smith, M.J.; Montagne, B.; Perrett, D.I.; Gill, M.; Gallagher, L. Detecting Subtle Facial Emotion Recognition Deficits in High-Functioning Autism Using Dynamic Stimuli of Varying Intensities. Neuropsychologia 2010, 48, 2777–2781. [Google Scholar] [CrossRef]
- Boucher, J.; Lewis, V. Unfamiliar Face Recognition in Relatively Able Autistic Children. J. Child Psychol. Psychiatry 1992, 33, 843–859. [Google Scholar] [CrossRef]
- Hobson, R.P.; Ouston, J.; Lee, A. What’s in a Face? The Case of Autism. Br. J. Psychol. 1988, 79, 441–453. [Google Scholar] [CrossRef]
- Riby, D.M.; Hancock, P.J.B. Viewing It Differently: Social Scene Perception in Williams Syndrome and Autism. Neuropsychologia 2008, 46, 2855–2860. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Tanaka, K.; Endo, Y.; Yamane, Y.; Yamamoto, T.; Nakano, Y.; Ohta, H.; Kato, N.; Kitazawa, S. Atypical Gaze Patterns in Children and Adults with Autism Spectrum Disorders Dissociated from Developmental Changes in Gaze Behaviour. Proc. R. Soc. B Biol. Sci. 2010, 277, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Leslie, A.M.; Frith, U. Does the Autistic Child Have a “Theory of Mind”? Cognition 1985, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L. Executive Dysfunction in Autism. Trends Cogn. Sci. 2004, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hedger, N.; Dubey, I.; Chakrabarti, B. Social Orienting and Social Seeking Behaviors in ASD. A Meta Analytic Investigation. Neurosci. Biobehav. Rev. 2020, 119, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Grelotti, D.J.; Gauthier, I.; Schultz, R.T. Social Interest and the Development of Cortical Face Specialization: What Autism Teaches Us about Face Processing. Dev. Psychobiol. 2002, 40, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Helt, M.; Kelley, E.; Kinsbourne, M.; Pandey, J.; Boorstein, H.; Herbert, M.; Fein, D. Can Children with Autism Recover? If So, How? Neuropsychol. Rev. 2008, 18, 339–366. [Google Scholar] [CrossRef]
- Lozier, L.M.; Vanmeter, J.W.; Marsh, A.A. Impairments in facial affect recognition associated with autism spectrum disorders: A meta-analysis. Dev. Psychopathol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Tomasello, M.; Camaioni, L. A Comparison of the Gestural Communication of Apes and Human Infants. Hum. Dev. 1997, 40, 7–24. [Google Scholar] [CrossRef]
- Auhagen, A.E.; von Salisch, M. The Diversity of Human Relationships; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-47983-7. [Google Scholar]
- Kanner, L. Early Infantile Autism. J. Pediatr. 1944, 25, 211–217. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The Autistic Mind: The Empathizing-Systemizing Theory. In Textbook of Autism Spectrum Disorders; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2011; pp. 39–47. ISBN 978-1-58562-341-9. [Google Scholar]
- Wakabayashi, A.; Baron-Cohen, S.; Uchiyama, T.; Yoshida, Y.; Kuroda, M.; Wheelwright, S. Empathizing and Systemizing in Adults with and without Autism Spectrum Conditions: Cross-Cultural Stability. J. Autism Dev. Disord. 2007, 37, 1823–1832. [Google Scholar] [CrossRef]
- Miralles, A.; Grandgeorge, M.; Raymond, M. Self-Perceived Empathic Abilities of People with Autism towards Living Beings Mostly Differs for Humans. Sci. Rep. 2022, 12, 6300. [Google Scholar] [CrossRef]
- Cross, L.; Farha, M.; Atherton, G. The Animal in Me: Enhancing Emotion Recognition in Adolescents with Autism Using Animal Filters. J. Autism Dev. Disord. 2019, 49, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Hilvert, E.; Misiunaite, I.; Kerby, K.; Giordano, M. Recognition of Facial Emotions on Human and Canine Faces in Children with and without Autism Spectrum Disorders. Motiv. Emot. 2019, 43, 191–202. [Google Scholar] [CrossRef]
- Gross, T.F. Perception of Human and Nonhuman Facial Age by Developmentally Disabled Children. J. Autism Dev. Disord. 2002, 32, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Atherton, G.; Cross, L. Seeing More Than Human: Autism and Anthropomorphic Theory of Mind. Front. Psychol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Amestoy, A. La Perception des Visages dans la Population Typique et les Troubles du Spectre Autistique (TSA). Ph.D. Thesis, Université de BORDEAUX 2, Bordeaux, France, 2013. [Google Scholar]
- Feng, S.; Wang, X.; Wang, Q.; Fang, J.; Wu, Y.; Yi, L.; Wei, K. The Uncanny Valley Effect in Typically Developing Children and Its Absence in Children with Autism Spectrum Disorders. PLoS ONE 2018, 13, e0206343. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Da Fonseca, D.; Esteves, F.; Deruelle, C. Motivational Approach and Avoidance in Autism Spectrum Disorder: A Comparison between Real Photographs and Cartoons. Res. Autism Spectr. Disord. 2015, 17, 13–24. [Google Scholar] [CrossRef]
- Saitovitch, A.; Bargiacchi, A.; Chabane, N.; Phillipe, A.; Brunelle, F.; Boddaert, N.; Samson, Y.; Zilbovicius, M. Studying Gaze Abnormalities in Autism: Which Type of Stimulus to Use? Open J. Psychiatry 2013, 3, 29770. [Google Scholar] [CrossRef]
- Brosnan, M.; Johnson, H.; Grawmeyer, B.; Chapman, E.; Benton, L. Emotion Recognition in Animated Compared to Human Stimuli in Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.; Piovesan, A.; Atherton, G. Autistic People Outperform Neurotypicals in a Cartoon Version of the Reading the Mind in the Eyes. Autism Res. 2022, 15, 1603–1608. [Google Scholar] [CrossRef]
- Pennisi, P.; Tonacci, A.; Tartarisco, G.; Billeci, L.; Ruta, L.; Gangemi, S.; Pioggia, G. Autism and Social Robotics: A Systematic Review. Autism Res. Off. J. Int. Soc. Autism Res. 2016, 9, 165–183. [Google Scholar] [CrossRef]
- Hernandez, N.; Metzger, A.; Magné, R.; Bonnet-Brilhault, F.; Roux, S.; Barthelemy, C.; Martineau, J. Exploration of Core Features of a Human Face by Healthy and Autistic Adults Analyzed by Visual Scanning. Neuropsychologia 2009, 47, 1004–1012. [Google Scholar] [CrossRef]
- Montagner, H. L’enfant et les animaux familiers. Enfances Psy 2007, 35, 15–34. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Dollion, N. L’animal de Compagnie Dans la vie des Enfants Au Développement Typique et Atypique et de Leur Famille. Rev. Int. L’éducation Fam. 2022, 1, 157–184. [Google Scholar]
- Byström, K.M.; Persson, C.A.L. The Meaning of Companion Animals for Children and Adolescents with Autism: The Parents’ Perspective. Anthrozoös 2015, 28, 263–275. [Google Scholar] [CrossRef]
- Redefer, L.A.; Goodman, J.F. Brief Report: Pet-Facilitated Therapy with Autistic Children. J. Autism Dev. Disord. 1989, 19, 461–467. [Google Scholar] [CrossRef]
- Carlisle, G.K. Pet Dog Ownership in Families of Children with Autism: Children’s Social Skills and Attachment to Their Dogs; University of Missouri-Columbia: Columbia, MO, USA, 2012. [Google Scholar]
- Celani, G. Human Beings, Animals and Inanimate Objects: What Do People with Autism Like? Autism 2002, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Prothmann, A.; Ettrich, C.; Prothmann, S. Preference for, and Responsiveness to, People, Dogs and Objects in Children with Autism. Anthrozoös 2009, 22, 161–171. [Google Scholar] [CrossRef]
- Maurer, M.; Delfour, F.; Trudel, M.; Adrien, J.-L. L’enfant avec un autisme et l’animal dans un lien signifiant: Des possibilités d’interventions thérapeutiques. Psychiatr. Enfant 2011, 54, 575–609. [Google Scholar] [CrossRef]
- Carlisle, G.K. Pet Dog Ownership Decisions for Parents of Children with Autism Spectrum Disorder. J. Pediatr. Nurs. 2014, 29, 114–123. [Google Scholar] [CrossRef]
- Grandgeorge, M. Les apports de l’animal dans le développement des enfants aux troubles du spectre autistique. Sens-Dessous 2015, 16, 17–26. [Google Scholar] [CrossRef]
- Grandgeorge, M. Communication Between Humans: Towards an Interdisciplinary Model of Intercomprehension. In Human-Robot Interaction: Evaluation Methods and Their Standardization; Jost, C., Le Pévédic, B., Belpaeme, T., Bethel, C., Chrysostomou, D., Crook, N., Grandgeorge, M., Mirnig, N., Eds.; Springer Series on Bio- and Neurosystems; Springer: Cham, Switzerland, 2020; pp. 3–19. ISBN 978-3-030-42307-0. [Google Scholar]
- Grandgeorge, M.; Degrez, C.; Alavi, Z.; Lemonnier, E. Face Processing of Animal and Human Static Stimuli by Children with Autism Spectrum Disorder: A Pilot Study. Hum.-Anim. Interact. Bull. 2016, 4, 39–53. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Sasson, N.J.; Reznick, J.S.; Paul, G.; Goldman, B.D.; Piven, J. Visual Scanning of Faces in Autism. J. Autism Dev. Disord. 2002, 32, 249–261. [Google Scholar] [CrossRef]
- Dollion, N.; Toutain, M.; François, N.; Champagne, N.; Plusquellec, P.; Grandgeorge, M. Visual Exploration and Observation of Real-Life Interactions between Children with ASD and Service Dogs. J. Autism Dev. Disord. 2021, 51, 3785–3805. [Google Scholar] [CrossRef] [PubMed]
- Uccheddu, S.; Albertini, M.; Pierantoni, L.; Fantino, S.; Pirrone, F. The Impacts of a Reading-to-Dog Programme on Attending and Reading of Nine Children with Autism Spectrum Disorders. Animals 2019, 9, 491. [Google Scholar] [CrossRef]
- Valiyamattam, G.J.; Katti, H.; Chaganti, V.K.; O’Haire, M.E.; Sachdeva, V. Do Animals Engage Greater Social Attention in Autism? An Eye Tracking Analysis. Front. Psychol. 2020, 11, 727. [Google Scholar] [CrossRef]
- Dollion, N.; Grandgeorge, M.; Saint-Amour, D.; Hosein Poitras Loewen, A.; François, N.; Fontaine, N.M.G.; Champagne, N.; Plusquellec, P. Emotion Facial Processing in Children With Autism Spectrum Disorder: A Pilot Study of the Impact of Service Dogs. Front. Psychol. 2022, 13, 869452. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L. Autistic Disturbances of Affective Contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- New, J.J.; Schultz, R.T.; Wolf, J.; Niehaus, J.L.; Klin, A.; German, T.C.; Scholl, B.J. The Scope of Social Attention Deficits in Autism: Prioritized Orienting to People and Animals in Static Natural Scenes. Neuropsychologia 2010, 48, 51–59. [Google Scholar] [CrossRef]
- McPartland, J.C.; Webb, S.J.; Keehn, B.; Dawson, G. Patterns of Visual Attention to Faces and Objects in Autism Spectrum Disorder. J. Autism Dev. Disord. 2011, 41, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Grandgeorge, M.; Deleau, M.; Lemonnier, E.; Tordjman, S.; Hausberger, M. Children with Autism Encounter an Unfamiliar Pet: Application of the Strange Animal Situation Test. Interact. Stud. 2012, 13, 165–188. [Google Scholar] [CrossRef]
- O’Haire, M.; McKenzie, S.; Beck, A.; Slaughter, V. Social Behaviors Increase in Children with Autism in the Presence of Animals Compared to Toys. PLoS ONE 2013, 8, e57010. [Google Scholar] [CrossRef]
- Guillon, Q.; Hadjikhani, N.; Baduel, S.; Kruck, J.; Arnaud, M.; Roge, B. Both Dog and Human Faces Are Explored Abnormally by Young Children with Autism Spectrum Disorders. Neuroreport 2014, 25, 1237–1241. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Bourreau, Y.; Alavi, Z.; Lemonnier, E.; Tordjman, S.; Deleau, M.; Hausberger, M. Interest towards Human, Animal and Object in Children with Autism Spectrum Disorders: An Ethological Approach at Home. Eur. Child Adolesc. Psychiatry 2015, 24, 83–93. [Google Scholar] [CrossRef]
- Muszkat, M.; de Mello, C.; Munoz, P.; Lucci, T.; David, V.; Siqueira, J.; Otta, E. Face Scanning in Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder: Human versus Dog Face Scanning. Front. Psychiatry 2015, 6, 150. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Gautier, Y.; Brugaillères, P.; Tiercelin, I.; Jacq, C.; Lebret, M.-C.; Hausberger, M. Social Rivalry Triggers Visual Attention in Children with Autism Spectrum Disorders. Sci. Rep. 2017, 7, 10029. [Google Scholar] [CrossRef]
- Doherty, B.R.; Charman, T.; Johnson, M.H.; Scerif, G.; Gliga, T.; BASIS Team. Visual Search and Autism Symptoms: What Young Children Search for and Co-Occurring ADHD Matter. Dev. Sci. 2018, 21, e12661. [Google Scholar] [CrossRef]
- Strathearn, L.; Kim, S.; Bastian, D.A.; Jung, J.; Iyengar, U.; Martinez, S.; Goin-Kochel, R.P.; Fonagy, P. Visual Systemizing Preference in Children with Autism: A Randomized Controlled Trial of Intranasal Oxytocin. Dev. Psychopathol. 2018, 30, 511–521. [Google Scholar] [CrossRef]
- Germone, M.M.; Gabriels, R.L.; Guérin, N.A.; Pan, Z.; Banks, T.; O’Haire, M.E. Animal-Assisted Activity Improves Social Behaviors in Psychiatrically Hospitalized Youth with Autism. Autism Int. J. Res. Pract. 2019, 23, 1740–1751. [Google Scholar] [CrossRef]
- Gale, C.M.; Eikeseth, S.; Klintwall, L. Children with Autism Show Atypical Preference for Non-Social Stimuli. Sci. Rep. 2019, 9, 10355. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, A.; Sorcinelli, A.; Rahman, T.; Elbogen, R.; Curtin, S.; Vouloumanos, A. Shifting Preferences for Primate Faces in Neurotypical Infants and Infants Later Diagnosed with ASD. Autism Res. 2019, 12, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Grandgeorge, M.; Gautier, Y.; Bourreau, Y.; Mossu, H.; Hausberger, M. Visual Attention Patterns Differ in Dog vs. Cat Interactions With Children With Typical Development or Autism Spectrum Disorders. Front. Psychol. 2020, 11, 2047. [Google Scholar] [CrossRef] [PubMed]
- Avila-Alvarez, A.; Alonso-Bidegain, M.; De-Rosende-Celeiro, I.; Vizcaino-Cela, M.; Larraneta-Alcalde, L.; Torres-Tobio, G. Improving Social Participation of Children with Autism Spectrum Disorder: Pilot Testing of an Early Animal-Assisted Intervention in Spain. Health Soc. Care Community 2020, 28, 1220–1229. [Google Scholar] [CrossRef]
- Scheerer, N.E.; Birmingham, E.; Boucher, T.Q.; Iarocci, G. Attention Capture by Trains and Faces in Children with and without Autism Spectrum Disorder. PLoS ONE 2021, 16, e0250763. [Google Scholar] [CrossRef]
- Dollion, N.; Herbin, A.; Champagne, N.; Plusquellec, P.; Grandgeorge, M. Characterization of Children with Autism Spectrum Disorder’s Interactions with a Service Dog during Their First Encounter. Anthrozoos 2022, 35, 867–889. [Google Scholar] [CrossRef]
- Autisme et Autres Troubles Envahissants du Développement. Available online: https://www.has-sante.fr/jcms/c_935617/fr/autisme-et-autres-troubles-envahissants-du-developpement (accessed on 14 December 2019).
- Ben-Yosef, D.; Anaki, D.; Golan, O. Context Processing in Adolescents with Autism Spectrum Disorder: How Complex Could It Be? Autism Res. Off. J. Int. Soc. Autism Res. 2017, 10, 520–530. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Van der Stigchel, S.; Nijboer, T.C.W.; Cornelissen, T.H.W.; Husain, M. CancellationTools: All-in-One Software for Administration and Analysis of Cancellation Tasks. Behav. Res. Methods 2015, 47, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Blois-Heulin, C. Variability in Social Visual Attention in the Red-Capped Mangabey (Cercocebus Torquatus Torquatus) and the Grey-Cheeked Mangabey (Cercocebus Albigena Albigena). Folia Primatol. 1999, 70, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Speer, L.L.; Cook, A.E.; McMahon, W.M.; Clark, E. Face Processing in Children with Autism: Effects of Stimulus Contents and Type. Autism 2007, 11, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, T.; Jordan, S. A Case Study in the Dynamics of Autism. In Autistic Spectrum Disorders: Educational and Clinical Interventions; Wahlberg, T., Obiakor, F., Burkhardt, S., Rotatori, A.F., Eds.; Advances in Special Education; Emerald Group Publishing Limited: Bingley, UK, 2001; Volume 14, pp. 53–65. ISBN 978-0-7623-0818-7. [Google Scholar]
- Lewy, A.L.; Dawson, G. Social Stimulation and Joint Attention in Young Autistic Children. J. Abnorm. Child Psychol. 1992, 20, 555–566. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, M.; Plaisted, K. Enhanced Discrimination in Autism. Q. J. Exp. Psychol. Sect. A 2001, 54, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, C.; Gilchrist, I.D.; Bender, A. Embedded Figures Detection in Autism and Typical Development: Preliminary Evidence of a Double Dissociation in Relationships with Visual Search. Dev. Sci. 2005, 8, 344–351. [Google Scholar] [CrossRef]
- Blake, R.; Turner, L.M.; Smoski, M.J.; Pozdol, S.L.; Stone, W.L. Visual Recognition of Biological Motion Is Impaired in Children With Autism. Psychol. Sci. 2003, 14, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Takarae, Y.; Luna, B.; Minshew, N.J.; Sweeney, J.A. Visual Motion Processing and Visual Sensorimotor Control in Autism. J. Int. Neuropsychol. Soc. 2014, 20, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Martin, A.; Baker, C.I.; Baron-Cohen, S. Atypical Integration of Motion Signals in Autism Spectrum Conditions. PLoS ONE 2012, 7, e48173. [Google Scholar] [CrossRef]
- Vacas, J.; Antolí, A.; Sánchez-Raya, A.; Pérez-Dueñas, C.; Cuadrado, F. Visual Preference for Social vs. Non-Social Images in Young Children with Autism Spectrum Disorders. An Eye Tracking Study. PLoS ONE 2021, 16, e0252795. [Google Scholar] [CrossRef]
- Howard, K.; Katsos, N.; Gibson, J. Using Interpretative Phenomenological Analysis in Autism Research. Autism 2019, 23, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Corden, B.; Chilvers, R.; Skuse, D. Avoidance of Emotionally Arousing Stimuli Predicts Social–Perceptual Impairment in Asperger’s Syndrome. Neuropsychologia 2008, 46, 137–147. [Google Scholar] [CrossRef]
- Kliemann, D.; Dziobek, I.; Hatri, A.; Steimke, R.; Heekeren, H.R. Atypical Reflexive Gaze Patterns on Emotional Faces in Autism Spectrum Disorders. J. Neurosci. 2010, 30, 12281–12287. [Google Scholar] [CrossRef]
- Dalton, K.M.; Nacewicz, B.M.; Johnstone, T.; Schaefer, H.S.; Gernsbacher, M.A.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Gaze Fixation and the Neural Circuitry of Face Processing in Autism. Nat. Neurosci. 2005, 8, 519–526. [Google Scholar] [CrossRef]
- Jemel, B.; Mottron, L.; Dawson, M. Impaired Face Processing in Autism: Fact or Artifact? J. Autism Dev. Disord. 2006, 36, 91–106. [Google Scholar] [CrossRef]
- Spezio, M.L.; Adolphs, R.; Hurley, R.S.E.; Piven, J. Analysis of Face Gaze in Autism Using “Bubbles”. Neuropsychologia 2007, 45, 144–151. [Google Scholar] [CrossRef]
- Guo, K.; Tunnicliffe, D.; Roebuck, H. Human Spontaneous Gaze Patterns in Viewing of Faces of Different Species. Perception 2010, 39, 533–542. [Google Scholar] [CrossRef]
- Bal, E.; Harden, E.; Lamb, D.; Van Hecke, A.V.; Denver, J.W.; Porges, S.W. Emotion Recognition in Children with Autism Spectrum Disorders: Relations to Eye Gaze and Autonomic State. J. Autism Dev. Disord. 2010, 40, 358–370. [Google Scholar] [CrossRef]
- Dimberg, U.; Petterson, M. Facial Reactions to Happy and Angry Facial Expressions: Evidence for Right Hemisphere Dominance. Psychophysiology 2000, 37, 693–696. [Google Scholar] [CrossRef]
- Morrison, R.L.; Bellack, A.S. The Role of Social Perception in Social Skill. Behav. Ther. 1981, 12, 69–79. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kohshima, S. Unique Morphology of the Human Eye. Nature 1997, 387, 767–768. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kohshima, S. Unique Morphology of the Human Eye and Its Adaptive Meaning: Comparative Studies on External Morphology of the Primate Eye. J. Hum. Evol. 2001, 40, 419–435. [Google Scholar] [CrossRef]
- Papagiannopoulou, E.A.; Chitty, K.M.; Hermens, D.F.; Hickie, I.B.; Lagopoulos, J. A Systematic Review and Meta-Analysis of Eye-Tracking Studies in Children with Autism Spectrum Disorders. Soc. Neurosci. 2014, 9, 610–632. [Google Scholar] [CrossRef] [PubMed]
- Knowledge, G. Pet Ownership Global GfK Survey 2016. Available online: https://cdn2.hubspot.net/hubfs/2405078/cms-pdfs/fileadmin/user_upload/country_one_pager/nl/documents/global-gfk-survey_pet-ownership_2016.pdf (accessed on 3 February 2024).
- Hare, B.; Brown, M.; Williamson, C.; Tomasello, M. The Domestication of Social Cognition in Dogs. Science 2002, 298, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, Á.; Kubinyi, E.; Topál, J.; Gácsi, M.; Virányi, Z.; Csányi, V. A Simple Reason for a Big Difference: Wolves Do Not Look Back at Humans, but Dogs Do. Curr. Biol. 2003, 13, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Buttelmann, D.; Tomasello, M. Can Domestic Dogs (Canis familiaris) Use Referential Emotional Expressions to Locate Hidden Food? Anim. Cogn. 2013, 16, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.A.; Schmitt, K.; Barber, A.L.A.; Huber, L. Dogs Can Discriminate Emotional Expressions of Human Faces. Curr. Biol. 2015, 25, 601–605. [Google Scholar] [CrossRef]
- Albuquerque, N.; Guo, K.; Wilkinson, A.; Savalli, C.; Otta, E.; Mills, D. Dogs Recognize Dog and Human Emotions. Biol. Lett. 2016, 12, 20150883. [Google Scholar] [CrossRef]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-Gaze Positive Loop and the Coevolution of Human-Dog Bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Range, F.; Horn, L.; Viranyi, Z.; Huber, L. The Absence of Reward Induces Inequity Aversion in Dogs. Proc. Natl. Acad. Sci. USA 2009, 106, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, G.K.; Johnson, R.A.; Wang, Z.; Brosi, T.C.; Rife, E.M.; Hutchison, A. Exploring Human–Companion Animal Interaction in Families of Children with Autism. J. Autism Dev. Disord. 2020, 50, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, G.K. The Social Skills and Attachment to Dogs of Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Borgi, M.; Cirulli, F. Children’s Preferences for Infantile Features in Dogs and Cats. Hum.-Anim. Interact. Bull. 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Dosso, J.A.; Huynh, M.; Kingstone, A. I Spy without My Eye: Covert Attention in Human Social Interactions. Cognition 2020, 202, 104388. [Google Scholar] [CrossRef]
- Kylliäinen, A.; Hietanen, J.K. Skin Conductance Responses to Another Person’s Gaze in Children with Autism. J. Autism Dev. Disord. 2006, 36, 517–525. [Google Scholar] [CrossRef]
- Whyte, E.M.; Behrmann, M.; Minshew, N.J.; Garcia, N.V.; Scherf, K.S. Animal, but Not Human, Faces Engage the Distributed Face Network in Adolescents with Autism. Dev. Sci. 2016, 19, 306–317. [Google Scholar] [CrossRef]
- Burrows, K.E.; Adams, C.L. Challenges of Service-Dog Ownership for Families with Autistic Children: Lessons for Veterinary Practitioners. J. Vet. Med. Educ. 2008, 35, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Viau, R.; Arsenault-Lapierre, G.; Fecteau, S.; Champagne, N.; Walker, C.-D.; Lupien, S. Effect of Service Dogs on Salivary Cortisol Secretion in Autistic Children. Psychoneuroendocrinology 2010, 35, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Borgi, M.; Francia, N.; Alleva, E.; Cirulli, F. Use of Assistance and Therapy Dogs for Children with Autism Spectrum Disorders: A Critical Review of the Current Evidence. J. Altern. Complement. Med. 2013, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bibbo, J.; Rodriguez, K.E.; O’Haire, M.E. Impact of Service Dogs on Family Members’ Psychosocial Functioning. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 2019, 73, 7303205120p1–7303205120p11. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.; Kaczmarek, E.; Drake, D. Parental Perceptions of the Nature of the Relationship Children with Autism Spectrum Disorders Share with Their Canine Companion. J. Autism Dev. Disord. 2019, 49, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Lisk, C.; Lawson, L.M.; Vaduvathiriyan, P. The Impact of Animal Exposure for Children with ASD: A Scoping Review. Rev. J. Autism Dev. Disord. 2021, 8, 471–481. [Google Scholar] [CrossRef]
- Hart, L.A.; Hart, B.L.; Thigpen, A.P.; Willits, N.H.; Lyons, L.A.; Hundenski, S. Compatibility of Cats with Children in the Family. Front. Vet. Sci. 2018, 5, 278. [Google Scholar] [CrossRef]
- O’Haire, M.E.; McKenzie, S.J.; McCune, S.; Slaughter, V. Effects of Classroom Animal-Assisted Activities on Social Functioning in Children with Autism Spectrum Disorder. J. Altern. Complement. Med. 2014, 20, 162–168. [Google Scholar] [CrossRef]
- O’Haire, M.E. Animal-Assisted Intervention for Autism Spectrum Disorder: A Systematic Literature Review. J. Autism Dev. Disord. 2013, 43, 1606–1622. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Hausberger, M. Autisme, médiation équine et bien-être. Bull. Académie Vét. Fr. 2019, 172, 47–53. [Google Scholar] [CrossRef]
- Filiâtre, J.C.; Millot, J.L.; Montagner, H. New Data on Communication Behaviour between the Young Child and His Pet Dog. Behav. Process. 1986, 12, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Martineau, J.; Cochin, S. Visual Perception in Children: Human, Animal and Virtual Movement Activates Different Cortical Areas. Int. J. Psychophysiol. 2003, 51, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Klin, A.; Lin, D.J.; Gorrindo, P.; Ramsay, G.; Jones, W. Two-Year-Olds with Autism Orient to Non-Social Contingencies Rather than Biological Motion. Nature 2009, 459, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.H.; Scott Young, W. Oxytocin: The Great Facilitator of Life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Quattrocki, E.; Friston, K. Autism, Oxytocin and Interoception. Neurosci. Biobehav. Rev. 2014, 47, 410–430. [Google Scholar] [CrossRef]
- Hollander, E.; Bartz, J.; Chaplin, W.; Phillips, A.; Sumner, J.; Soorya, L.; Anagnostou, E.; Wasserman, S. Oxytocin Increases Retention of Social Cognition in Autism. Biol. Psychiatry 2007, 61, 498–503. [Google Scholar] [CrossRef]
- Guastella, A.J.; Einfeld, S.L.; Gray, K.M.; Rinehart, N.J.; Tonge, B.J.; Lambert, T.J.; Hickie, I.B. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biol. Psychiatry 2010, 67, 692–694. [Google Scholar] [CrossRef]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The Effect of Oxytocin Nasal Spray on Social Interaction Deficits Observed in Young Children with Autism: A Randomized Clinical Crossover Trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Hurlemann, R.; Patin, A.; Onur, O.A.; Cohen, M.X.; Baumgartner, T.; Metzler, S.; Dziobek, I.; Gallinat, J.; Wagner, M.; Maier, W.; et al. Oxytocin Enhances Amygdala-Dependent, Socially Reinforced Learning and Emotional Empathy in Humans. J. Neurosci. 2010, 30, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Andari, E.; Duhamel, J.-R.; Zalla, T.; Herbrecht, E.; Leboyer, M.; Sirigu, A. Promoting Social Behavior with Oxytocin in High-Functioning Autism Spectrum Disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 4389–4394. [Google Scholar] [CrossRef] [PubMed]
- Yamasue, H.; Domes, G. Oxytocin and Autism Spectrum Disorders. In Behavioral Pharmacology of Neuropeptides: Oxytocin; Hurlemann, R., Grinevich, V., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2018; pp. 449–465. ISBN 978-3-319-63739-6. [Google Scholar]
- Benner, S.; Yamasue, H. Clinical Potential of Oxytocin in Autism Spectrum Disorder: Current Issues and Future Perspectives. Behav. Pharmacol. 2018, 29, 1–12. [Google Scholar] [CrossRef]
- Nagasawa, M.; Kikusui, T.; Onaka, T.; Ohta, M. Dog’s Gaze at Its Owner Increases Owner’s Urinary Oxytocin during Social Interaction. Horm. Behav. 2009, 55, 434–441. [Google Scholar] [CrossRef]
- Powell, L.; Guastella, A.J.; McGreevy, P.; Bauman, A.; Edwards, K.M.; Stamatakis, E. The Physiological Function of Oxytocin in Humans and Its Acute Response to Human-Dog Interactions: A Review of the Literature. J. Vet. Behav. 2019, 30, 25–32. [Google Scholar] [CrossRef]
- Serpell, J.A. Factors Influencing Human Attitudes to Animals and Their Welfare. Anim. Welf. 2004, 13, S145–S151. [Google Scholar] [CrossRef]
- Borgi, M.; Cogliati-Dezza, I.; Brelsford, V.; Meints, K.; Cirulli, F. Baby Schema in Human and Animal Faces Induces Cuteness Perception and Gaze Allocation in Children. Front. Psychol. 2014, 5, 411. [Google Scholar] [CrossRef]
- Kiani, R.; Bhaumik, S.; Tyrer, F.; Bankart, J.; Miller, H.; Cooper, S.A.; Brugha, T.S. The Relationship between Symptoms of Autism Spectrum Disorder and Visual Impairment among Adults with Intellectual Disability. Autism Res. 2019, 12, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric Disorders in Children with Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Cavézian, C.; Vilayphonh, M.; de Agostini, M.; Vasseur, V.; Watier, L.; Kazandjian, S.; Laloum, L.; Chokron, S. Assessment of Visuo-Attentional Abilities in Young Children with or without Visual Disorder: Toward a Systematic Screening in the General Population. Res. Dev. Disabil. 2010, 31, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Vidal. Available online: https://www.vidal.fr/ (accessed on 3 June 2023).
- Enquete Facco/Kantar. Facco Plus D’un Français Sur Deux Possede Un Animal De Compagnie! 2021. Available online: https://www.facco.fr/chiffres-cles/les-chiffres-de-la-population-animale/ (accessed on 3 February 2024).
- Amici, F.; Waterman, J.; Kellermann, C.M.; Karimullah, K.; Bräuer, J. The Ability to Recognize Dog Emotions Depends on the Cultural Milieu in Which We Grow Up. Sci. Rep. 2019, 9, 16414. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T.; Johnson, C. Animals in Translation: Using the Mysteries of Autism to Decode Animal Behavior; SUNY Press: Albany, NY, USA, 2009; ISBN 978-1-4391-3084-1. [Google Scholar]

| Authors | Year | Publication Title | Article Title |
|---|---|---|---|
| New et al. [89] | 2010 | Neuropsychologia | The scope of social attention deficits in autism: prioritized orienting to people and animals in static natural scenes |
| McPartland et al. [90] | 2011 | Journal of Autism and Developmental Disorders | Patterns of visual attention to faces and objects in autism spectrum disorder |
| Grandgeorge et al. [91] | 2012 | Interaction Studies | Children with autism encounter an unfamiliar pet: application of the strange animal situation test |
| O’Haire et al. [92] | 2013 | PLoS ONE | Social behaviours increase in children with autism in the presence of animals compared to toys |
| Guillon et al. [93] | 2014 | Neuroreport | Both dog and human faces are explored abnormally by young children with autism spectrum disorders |
| Grandgeorge et al. [94] | 2015 | European Child and Adolescent Psychiartry | Interest towards human animals and objects of children with autism spectrum disorders: an ethological approach at home |
| Muszkat et al. [95] | 2015 | Frontiers in Psychiatry | Face scanning in autism spectrum disorder and attention deficit/hyperactivity disorder: human versus dog face scanning |
| Grandgeorge et al. [80] | 2016 | Human-Animal Interaction Bulletin | Face processing of animal and human static stimuli by children with autism spectrum disorder: a pilot study |
| Grandgeorge et al. [96] | 2017 | Scientific Reports | Social rivalry triggers visual attention in children with autism spectrum disorders |
| Doherty et al. [97] | 2018 | Developmental Science | Visual search and autism symptoms: what young children search for and co-occurring ADHD matter |
| Strathearn et al. [98] | 2018 | Development and Psychopathology | Visual systemizing preference in children with autism: A randomized controlled trial of intranasal oxytocin |
| Germone et al. [99] | 2019 | Autism | Animal-assisted activity improves social behaviours in psychiatrically hospitalized youths with autism |
| Uccheddu et al. [83] | 2019 | Animals | The impacts of a reading-to-dog programme on attending and reading of nine children with autism spectrum disorders |
| Gale et al. [100] | 2019 | Scientific Reports | Children with autism show atypical preference for non-social stimuli |
| Yamashiro et al. [101] | 2019 | Autism Research | Shifting preferences for primate faces of neurotypical infants and infants later diagnosed with ASD |
| Grandgeorge et al. [102] | 2020 | Frontiers in Psychology | Visual attention patterns differ in dog vs. cat interactions with children with typical development or autism spectrum disorders |
| Avila-Alvarez et al. [103] | 2020 | Health and Social Care in the Community | Improving social participation of children with autism spectrum disorder: pilot testing of an early animal-assisted intervention in Spain |
| Valiyamattam et al. [84] | 2020 | Frontiers in Psychology | Do animals engage greater social attention in autism? An eye tracking analysis |
| Dollion et al. [82] | 2021 | Journal of Autism and Developmental Disorders | Visual exploration and observation of real-life interactions between children with ASD and service dogs |
| Scheerer et al. [104] | 2021 | PloS ONE | Attention capture by trains and faces in children with and without autism spectrum disorder |
| Dollion et al. [105] | 2022 | Anthrozoos | Characterization of children with autism spectrum disorder’s interactions with a service dog during their first encounter |
| Authors | N ASD | Diagnoses | Diagnostic Tools | Additional Tools for ASD Measurement | Age of ASD Subjects (Mean ± SD)/(Range) | Gender of ASD Subjects | Pet Ownership |
|---|---|---|---|---|---|---|---|
| New et al. [89] | 31 | ASD | ADI-R, ADOS | VABS | 10.8 ± 3.4 yo | 30 M; 1 F | NA |
| McPartland et al. [90] | 15 | ASD | ADI-R ADOS clinical diagnosis DSM-IV-TR criteria for AS | The VABS—Survey Form based on parent reports, the Social Competence Questionnaire, and SRS | 14.5 ± 1.7 yo (12.0–16.6) | 13 M; 2 F | NA |
| Grandgeorge et al. [91] | 27 | ASD | ADI-R, DSM-IV criteria | CARS, Vineland for a part of them. | 9.6 ± 1.8 yo (6–12) | 27 M | 19 with pets |
| O’Haire et al. [92] | 33 | ASD (n = 7) AS (n = 14) PDD-NOS (n = 5) and AD (n = 7) | All had a previous diagnosis established by experts | SCQ and SSRS for a part of them completed by parent and teacher | 9.4 ± 2.3 yo (5.2–12.1) | 24 M; 9 F | All ASD children |
| Guillon et al. [93] | 19 | ASD | ADOS-G and ADI-R performed by at least one clinical psychologist | Mullen Scales of Early Learning | 39.6 ± 10.5 months (24–60) | 15 M; 4 F | NA |
| Grandgeorge et al. [94] | 31 | ASD | The ADI-R by independent psychiatrists | VABS, CARS, and ICD-10 criteria | 9.5 ± 1.8 yo (6–12) | 30 M; 1 F | 22 with pets |
| Muszkat et al. [95] | 15 | ASD | CARS and DSM-V criteria | NA | 11.6 ± 2.7 yo | 13 M; 2 F | NA |
| Grandgeorge et al. [80] | 12 | AD (n = 3) AS (n = 9) | ADI-R, ADOS | NA | 136.6 ± 14.7 months (87–187) | 10 M; 2 F | Some with pets, but number of concerned participants not specified |
| Grandgeorge et al. [96] | S1: 20 S2: 9 | ASD | S1 and S2: A parent-reported diagnosis of ASD and a medical report of ASD using ADI-R1, and DSM-IV criteria | SCQ completed by parents | S1: 7.6 ± 1.6 yo S2: 13.7 ± 2.3 yo | S1: 9 M; 1 F S2: 8 M; 1 F | NA |
| Doherty et al. [97] | 98 HR | 56 HR Typical 28 HR Atypical 14 HR ASD | ADOS-2, ADI-R, and DSM-5 criteria. | Vineland-II, Mullen Scales of Early Learning, SBRI | 38.8 ± 1.57 months | 55 M; 43 F | NA |
| Strathearn et al. [98] | 16 | ASD | ADI-R, ADOS, and DSM-IV criteria | VABS | 12.8 ± 3.4 yo (8.2–19.0) | 16 M | NA |
| Germone et al. [99] | 67 | ASD | ADOS-2 criteria | SCQ | 11.7 ± 3.5 yo | 53 M; 13 F; 1 NA | 23 with dogs, 15 with cats, 1 with rabbits, 1 with guinea pigs, 15 with multiple types of pets |
| Uccheddu et al. [83] | 5 | ASD | DSM-V criteria and tools required by the Ministry of Health | NA | 7.60 ± 2.3 yo (SE) (6–11) | 4 M; 1 F | NA |
| Gale et al. [100] | S2: 19 | ASD | ICD-10 criteria | CARS-2, VABS | S2: 58.2 ± 21.1 months (26–96) | S2: 15 M; 4 F | None of the participants had pets |
| Yamashiro et al. [101] | S1: 15 ASD (according to the age of the children tested, N varies between 7 and 11). S2: 7 ASD (according to the age of the children tested, N varies between 5 and 7) | ASD | Toddler Module of ADOS | NA | S1 and S2: NA (6–18 months), test at 6 (only S1), 9, 12 months | NA | NA |
| Grandgeorge et al. [102] | 22 | ASD | ADI-R, DSM-IV criteria | ICD-10 criteria | 10.1 ± 2.1 yo (6–12) with a dog and 7.5 ± 2.2 yo (6–12) with a cat | 14 M | 14 with dogs, 8 with cats |
| Avila-Alvarez et al. [103] | 19 | ASD (n = 15), probable ASD (n = 4) | DSM V criteria | NA | 46.2 ±12.6 months (30–66) | 13 M; 6 F | 7 with dogs and 3 with cats |
| Valiyamattam et al. [84] | 21 | ASD | Parent and/or teacher reported diagnosis of ASD | SCQ, SRS-2 | 10.3 ± 1.60 yo (5–12) | 16 M; 5 F | NA |
| Dollion et al. [82] | S1: 16 S2: 6 | ASD | Parent reported diagnosis of ASD | CARS | S1: 8.5 ± 0.7 yo S2: 9.3 ± 1.1 yo (6–15) | S1: 14 M; 2 F S2: 3 M; 3 F | NA |
| Scheerer et al. [104] | S1: 29 S2: 10 | ASD | British Columbia (BC) clinical diagnostic report ADOS, ADI-R | AQ parent-report questionnaire | S1: 10.35 ± 1.93 yo (6–14) S2: 9.02 ± 2 yo (6–12) | S1: 20 M; 9 F S2: 8 M; 2 F | NA |
| Dollion et al. [105] | 20 | ASD | Parent reported diagnosis of ASD | NA | 8.6 ± 27 yo (3–12) | 16 M; 4 F | NA |
| Stimuli Characteristics | Results | ||||
|---|---|---|---|---|---|
| Authors | Species/Breed of Animals (n = ) | Type of Material (e.g., Screen Tablet, Real Interaction) | Animal Known or Unknown to the Subject | Main Significant Results about Visual Attention | Main Limits Mentioned |
| New et al. [89] | Color photographs of natural scenes; 14 scenes for the animal semantic category. Target animals were primates of the Catarrhini sub-order (n = 1); common Eland (n = 1), and pigeon (n = 1) | Computer screen SS: NA SD: NA | Unknown | Children with ASD exhibited robust social attentional biases for categorical animacy—detecting changes faster and more reliably in people and animals compared to artifacts and plants. | None were mentioned. |
| McPartland et al. [90] | Digitized standardized grayscale images: Monkey faces (n = 10) presented bilaterally and symmetrically | Computer screen SS: 21-inch SD: 15° × 19° covert | Unknown | Both groups devoted greater attention to the upper versus lower AOI for both human and monkey faces. No other effects or between-group differences were detected. | Study design limits inferential power and generalizability. Influence of the particular visual characteristics of a homogenous stimulus class on viewing patterns could not be explored. Wide range of participants’ age, developmental effects could create a bias. Measures of fixation were limited to theoretically-defined AOIs. Aspects of the experimental design may influence the results. Extended viewing times of 8 s may have affected viewing patterns. |
| Grandgeorge et al. [91] | Guinea pigs (n = 4) | Real interactions | Unknown | First gaze of the children with ASD upon entering the room with the guinea pig was mostly towards the animal with 66.7% of the children with ASD having looked at the animal. | None were mentioned. |
| O’Haire et al. [92] | Guinea pigs (n = 2 per observation; n = 30 in total) | Real interactions | Unknown at the beginning (8 weeks of experiment, twice-weekly session) | Children with ASD looked at the toys significantly more often than at the animals. | Lack of information about participants’ cognitive functioning or IQ. Difference in the availability of animals (n = 2) vs. toys (large variety). |
| Guillon et al. [93] | Grayscale photographs: dog faces (n = 12) with negatively (n = 4), neutrally (n = 4), and positively (n = 4) valenced expressions | Computer screen SS: 17-inch SD: 19° × 20° covered | Unknown | There was no gaze bias to the left visual hemifield for the ASD group (whether human or canine), while the TD group manifested a left bias for both species. Emotional valence had no effect on the laterality index of the first fixation for both human and canine faces. | Children’s manual and ocular dominances were not evaluated. |
| Grandgeorge et al. [94] | Guinea pig (n = 4) | Real interactions | Unknown | TD children performed more visual behaviors towards the animal compared to ASD children (eye direction toward the animal: 48.8 ± 17.2% vs. 79.7 ± 9.6%, respectively). Children with ASD looked more at the animal than they touched it. The visual index of focusing only on the pet was higher for TD children than for ASD children. | Limitation due to cross-sectional design. |
| Muszkat et al. [95] | Color photographs: dog faces with neutral expressions (n = NA) | Computer screen SS: 23-inch SD: NA | Unknown | All children looked more at dog pictures than at human ones. The eye area was looked at the longest compared to the mouth area in all children. The duration of fixation on the eyes was lower in the ASD group than in the TD group. When children with ASD looked at human and dog faces, they did not manifest the left visual hemifield preference observed in the TD group. | Small sample size, as well as the demographic and clinical heterogeneity of the samples. |
| Grandgeorge et al. [80] | Black-and-white pictures of dog (n = 2), cat (n = 2), and horse (n = 2) faces in a natural context | Computer screen SS: NA SD: resolution of 500 × 800 pixels | Unknown | Children with ASD spent less time looking at the eyes on all animal pictures than TD children. No difference between the two groups in time spent looking at the mouth or ears. All children looked longer at humans’ mouths than at animals’ mouths. Children with ASD looked more at the eyes than at the mouth and ears of dog pictures, and they looked longer at mouths than at ears. Children with ASD looked more at the eyes than at the mouths and ears of cat pictures. They looked more at the eyes and mouth than at the ears of the horse pictures. | Small sample size of the ASD group. Pictures were not standardized in terms of AOI sizes and/or background. A small number of pictures used. |
| Grandgeorge et al. [96] | S1: Service dogs (n = 9): three Labrador retrievers and six golden retrievers (eight males; mean age ± SD: 23.8 ± 0.5 months) S2: Service dogs (n = 2): one male Labrador retriever and one female golden retriever (mean age ± SD: 23.9 ± 0.2 months) | Real interactions | Unknown | S1: children with ASD alternate more glances between the service dog and the dog trainer when the child is out of the service dog-animal trainer interactions. Compared to the control group, children with ASD glanced more towards the dog-trainer dyad when they both had a close interaction without visually attending to the child. S2: the three repetitions of the procedure showed that children with ASD maintained strong visual attention towards the service dog-trainer dyad when the child was out of the interaction. Most instances of joint attention between children with ASD and the trainer were on the service dog (85%). | None was mentioned. |
| Doherty et al. [97] | Colored pictures of animals: bears, camels, cats, cows, and dogs (same picture for each species, n = NA) | Touchscreen monitor. SS: 14.94 × 11.94-inch SD: 3.18 cm × 2.39 cm | Unknown | In multi-target cancellation tasks with more complex targets/distractors, ASD symptoms were associated with more disorganized visual search across conditions and poorer search performance, for categorical search in particular. ASD symptom severity did not correlate with search performance, but it did correlate with poorer categorical search. | Diagnosis is not confirmed with a gold standard measure. The three experimental conditions were administered in a fixed order, allowing for potential order or fatigue bias. Presence of missing data for 10 children. Small sample size. |
| Strathearn et al. [98] | four images of four levels of picture systemizing (systematization is the level of organization of the image (e.g., a high level of systematization means that the elements in the scene are highly organized)) positioned in each quadrant of each slide. Systemizing Picture Task using real-life images of animals (not specified, but provide examples of images with anseriform birds (n = 4)) | Computer screen SS: 17-inch SD: NA | Unknown | In the placebo condition, children with ASD showed a visual preference for more highly systemized images. There was a significant linear trend for an increase in the duration of fixation in children with ASD with increasing levels of picture systemizing, while no such effect was seen in the control group. | Systemizing Picture Task has not yet been validated. Physical aspects of the pictures, such as color intensity or background features, were not controlled for and may have influenced gaze preference. Lack of systematic information on psychiatric comorbidity. |
| Germone et al. [99] | Therapy dogs (n = 6; all females): golden retriever (n = 2), Border Collie/golden retriever Mix(n = 1), King Charles Spaniel Mix (n = 1) and Labrador Mix (n = 2) | Real interactions | Unknown at the beginning (NA total session, two sessions per week) | Children with ASD in the control condition looked more at the toy; children with ASD in the experimental condition looked more at the dog. | Participants were not randomly assigned to experimental (AAA with a dog) or control (toy) conditions. Intra-individual variability of participant’s behaviors during their hospital stay. |
| Uccheddu et al. [83] | Therapy dogs (n = 2): 2 females, mixed-breed dogs | Real interactions | Unknown at the beginning (10 weekly sessions for the experimental group) | Questions to parents concerning their child’s “attention to dogs” in their daily lives revealed no differences between children with ASD in the experimental condition (reading in the presence of a dog) or the control condition (reading without the dog). | Small sample size. The effects of confounding variables (e.g., parenting styles, comorbid outcomes such as anxiety) were not controlled for. Subjectivity due to parental report. Potential desirability bias in the answers to the questionnaire. |
| Gale et al. [100] | Videos: dogs’ faces (n = 6) | Tablet screen SS: NA SD: 7 cm × 7 cm, and after touch, 13 × 13 cm. | Unknown | Children with ASD preferred non-social stimuli (geometric images) to dog faces (geometric images were indicated as preferred 69.9 ± 14.8% of the time compared to 52% for dog faces). Preference for non-social stimuli remained high for children with ASD, while it decreased for TD children across sessions. | Many of the same participants took part in the three studies. There was no cognitive assessment of participants. Human stimuli were only Caucasian adults, while the participants had various ethnicities. |
| Yamashiro et al. [101] | Grayscale photographs placed on a gray background: adult female rhesus monkey faces (n = 2) | Computer screen SS: 29 cm × 47 cm (equivalent 22-inch). SD: 16.5 × 20 cm (adjusted to screen size) | Unknown | 6-month-old infants later diagnosed with ASD only preferred human faces over non-faces and did not prefer monkey faces over non-faces until reaching 9 months of age. Compared to TD infants, infants later diagnosed with ASD showed a greater downturn in their preference for primate faces compared to non-faces, as well as in their total looking time for both primate faces between 6 and 18 months. | None mentioned. |
| Grandgeorge et al. [102] | Dogs (n = 14) (six females) Cats (n = 8) (five females) | Real interactions and questionnaires | Known | Mutual gazes with cats were rare between children of both groups. In both groups, cats and children initiated glances and mutual gazes equally often. Children with ASD directed more gazes and glances towards their cats than their dogs. Parents of both groups reported that their children had fewer visual interactions with cats than with dogs. | The length of the videos varied due to the ecological situation. |
| Avila-Alvarez et al. [103] | Therapy dogs (n = 5; four males and one female): Labrador retrievers (n = 3), Galician Shepherd Dog (n = 1), Spanish Water Dog breed (n = 1) | Real interactions | Unknown at the beginning (total of 9 weekly sessions) | Significant increase in the frequency of children with ASD’s eye contacts with the dog and in their participation in activities with the dog across sessions. | Absence of a control group. Lack of information, such as the severity of ASD. Non-probability convenience sample (Sample of participants not representative of the whole population (i.e., non-random); here for example, the sample was recruited by a non-random technique and participants were predominantly male). Inclusion of children with probable ASD. Variability in the number and length of AAI sessions. |
| Valiyamattam et al. [84] | Color photographs of animals against a constant gray backdrop: dogs (n = 8), cats (n = 8), horses (n = 2) and cows (n = 2) | Computer screen SS: 21.5-inch SD: 29.5° × 32.5° | Unknown | Children with ASD showed significantly less visual attention to the face and eye area, left and right eyes, as well as to the mouth area on both human and animal pictures combined compared to TD children. Children with ASD showed greater visual attention to the face and eye region of animal pictures compared to human pictures. Children with ASD showed greater visual attention toward the mouth and screen areas on human pictures compared to animal pictures. Children with ASD showed greater visual attention to the eye regions and face of animals for front-facing pictures compared to averted-facing images. No significant difference was observed in TD and ASD children’s visual attention to the mouth area between front and averted-facing human and animal pictures. | ASD children’s mental age was lower than that of TD children. The number of males was higher in the children with ASD group. |
| Dollion et al. [82] | S1: Service dogs (n = 15; 10 females): Labradors (n = 5) Labernois (n = 3) and Saint-Pierre (n = 5) S2: Service dogs (n = 4; all females): Labradors (n = 2), Labernois (n = 1), Saint-Pierre (n = 1) | Real interactions | Unknown | Children with ASD spend more time looking at the service dog, particularly its head, than at other elements of the visual scene (mean percentage of fixation duration: 44.4 ± 3.2% on the service dog and 31.4 ± 2.5% on the service dog’s head). Some of the ASD children’s interaction behaviors correlate with their visual exploration (e.g., attention to animate/inanimate stimuli). Children with ASD who looked more at the social/animate targets showed more engagement in their interaction with the service dog. | Small sample size. Participant selection was partly based on their initial attraction towards dogs. Choice of AOIs for analysis (i.e., the decision applied to select and delimit AOIs may have had a limiting effect on the collected data). |
| Scheerer et al. [104] | S1 and S2, gray-scale pictures: butterfly (n = 1) and fish (n = 1) | Computer screen SS: 23-inch SD: 3 × 3 cm | S1 and S2: Unknown | When the target was absent, both groups of children were more accurate but slower at detecting the absence or presence of the butterfly target. For both groups, the butterfly target was fixated on the majority of targets present in the trials. Children with ASD detected the butterfly more slowly and took longer to fixate the butterfly than TD children. | Age of participants. Inter- and intra-individual variability with regard to past experience with the stimuli and interest in the stimuli. The task was too easy. Small sample of children for the eye-tracking part. |
| Dollion et al. [105] | Service dog (n = 18; 5 females): Labrador (n = 8), Labernois (n = 3), Saint-Pierre (n = 7) | Real interactions | Unknown | The service dog was the preferred target of ASD children’s gazes. Younger children with ASD gazed less at the service dog than older children. Two profiles of children with ASD were observed: one group interacted distally with the service dog and looked less at it compared to the second group, which interacted more proximally and looked more at it. | All participants were on the waiting list to receive a service dog (potential bias of attraction towards dogs). Narrow range of autism severity. Absence of information concerning the presence/absence of pets at home, previous experience with animals, and frequency of activities with animals. The first version of the CARS scale was used. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toutain, M.; Dollion, N.; Henry, L.; Grandgeorge, M. How Do Children and Adolescents with ASD Look at Animals? A Scoping Review. Children 2024, 11, 211. https://doi.org/10.3390/children11020211
Toutain M, Dollion N, Henry L, Grandgeorge M. How Do Children and Adolescents with ASD Look at Animals? A Scoping Review. Children. 2024; 11(2):211. https://doi.org/10.3390/children11020211
Chicago/Turabian StyleToutain, Manon, Nicolas Dollion, Laurence Henry, and Marine Grandgeorge. 2024. "How Do Children and Adolescents with ASD Look at Animals? A Scoping Review" Children 11, no. 2: 211. https://doi.org/10.3390/children11020211