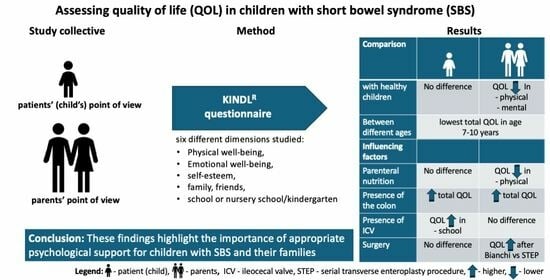
Quality of Life of Children with Short Bowel Syndrome from Patients’ and Parents’ Points of View
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment of Quality of Life with the KINDL Questionnaire
- “Kiddy” questionnaire for 4–6-year-old children;
- “Kid” questionnaire for 7–13-year-olds;
- “Kiddo” questionnaire for adolescents aged 14–17 years.
2.2. Healthy Population for Comparison
2.3. Statistical Methods
3. Results
3.1. Health-Related Quality of Life of the Patients with SBS
3.1.1. Comparison of the Quality of Life with Norm Values
3.1.2. Quality of Life Depending on Age
3.1.3. Differences in Age Grouping with Healthy Children
3.2. Influence of the Collected Parameters on the Total Quality of Life
3.2.1. Influence of the Type of Intestinal Lengthening Procedures on the Quality of Life
3.2.2. Influence of the Ileocecal Valve on Quality of Life
3.2.3. Influence of the Colon on the Quality of Life
3.2.4. Influence of Parenteral Nutrition on Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hukkinen, M.; Merras-Salmio, L.; Pakarinen, M.P. Health-related quality of life and neurodevelopmental outcomes among children with intestinal failure. Semin. Pediatr. Surg. 2018, 27, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Berghöfer, P.; Fragkos, K.C.; Baxter, J.P.; Forbes, A.; Joly, F.; Heinze, H.; Loth, S.; Pertkiewicz, M.; Messing, B.; Jeppesen, P.B. Development and validation of the disease-specific Short Bowel Syndrome-Quality of Life (SBS-QoL) scale. Clin. Nutr. 2013, 32, 789–796. [Google Scholar] [CrossRef]
- Kelly, D.G.; Tappenden, K.A.; Winkler, M.F. Short bowel syndrome: Highlights of patient management, quality of life, and survival. J. Parenter. Enter. Nutr. 2014, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.F.; Smith, C.E. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. J. Parenter. Enter. Nutr. 2014, 38, 32s–37s. [Google Scholar] [CrossRef] [PubMed]
- Ravens-Sieberer, U.; Bullinger, M. Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Qual. Life Res. 1998, 7, 399–407. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Ellert, U.; Erhart, M. Health-related quality of life of children and adolescents in Germany. Norm data from the German Health Interview and Examination Survey (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 810–818. [Google Scholar] [CrossRef] [PubMed]
- KINDL. Available online: https://www.kindl.org/english/analysis/evaluation-using-spss/ (accessed on 12 March 2024).
- Starfield, B.; Riley, A.W.; Green, B.F.; Ensminger, M.E.; Ryan, S.A.; Kelleher, K.; Kim-Harris, S.; Johnston, D.; Vogel, K. The adolescent child health and illness profile. A population-based measure of health. Med. Care 1995, 33, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Ravens-Sieberer, U.; Herdman, M.; Devine, J.; Otto, C.; Bullinger, M.; Rose, M.; Klasen, F. The European KIDSCREEN approach to measure quality of life and well-being in children: Development, current application, and future advances. Qual. Life Res. 2014, 23, 791–803. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Rode, C.A. The PedsQL: Measurement model for the pediatric quality of life inventory. Med. Care 1999, 37, 126–139. [Google Scholar] [CrossRef]
- Fuerboeter, M.; Boettcher, J.; Barkmann, C.; Zapf, H.; Nazarian, R.; Wiegand-Grefe, S.; Reinshagen, K.; Boettcher, M. Quality of life and mental health of children with rare congenital surgical diseases and their parents during the COVID-19 pandemic. Orphanet J. Rare Dis. 2021, 16, 498. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Hauck, M.; Fuerboeter, M.; Elrod, J.; Vincent, D.; Boettcher, J.; Reinshagen, K. Clinical outcome, quality of life, and mental health in long-gap esophageal atresia: Comparison of gastric sleeve pull-up and delayed primary anastomosis. Pediatr. Surg. Int. 2023, 39, 166. [Google Scholar] [CrossRef] [PubMed]
- Baars, R.M.; I Atherton, C.; Koopman, H.M.; Bullinger, M.; Power, M.; The DISABKIDS Group. The European DISABKIDS project: Development of seven condition-specific modules to measure health related quality of life in children and adolescents. Health Qual. Life Outcomes 2005, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Kenzik, K.M.; Tuli, S.Y.; Revicki, D.A.; Shenkman, E.A.; Huang, I.C. Comparison of 4 Pediatric Health-Related Quality-of-Life Instruments: A Study on a Medicaid Population. Med. Decis. Mak. 2014, 34, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Ravens-Sieberer, U.; Karow, A.; Barthel, D.; Klasen, F. How to assess quality of life in child and adolescent psychiatry. Dialogues Clin. Neurosci. 2014, 16, 147–158. [Google Scholar] [CrossRef]
- Körner, I.; Schlüter, C.; Lax, H.; Rübben, H.; Radmayr, C. Health-related quality of life in children with spina bifida. Urologe 2006, 45, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Straßburger, K.; Lange, K.; Bächle, C.; Holl, R.W.; Giani, G.; Rosenbauer, J. Health-related quality of life among German youths with early-onset and long-duration type 1 diabetes. Diabetes Care 2012, 35, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Hövels-Gürich, H.H.; Konrad, K.; Skorzenski, D.; Minkenberg, R.; Herpertz-Dahlmann, B.; Messmer, B.J.; Seghaye, M.-C. Long-term behavior and quality of life after corrective cardiac surgery in infancy for tetralogy of Fallot or ventricular septal defect. Pediatr. Cardiol. 2007, 28, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Musial-Bright, L.; Panteli, L.; Driever, P.H. Pediatric low-grade glioma survivors experience high quality of life. Childs Nerv. Syst. 2011, 27, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.C.; Steiner, K.; Wild, J.M.; Stark, V.; Kozlik-Feldmann, R.; Mir, T.S. Health-related quality of life is unimpaired in children and adolescents with Marfan syndrome despite its distinctive phenotype. Acta Paediatr. 2016, 105, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Kosola, S.; Merras-Salmio, L.; Kolho, K.-L.; Pakarinen, M.P. Long-term health-related quality of life of patients with pediatric onset intestinal failure. J. Pediatr. Surg. 2015, 50, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Sudan, D.; Horslen, S.; Botha, J.; Grant, W.; Torres, C.; Shaw, B.; Langnas, A. Quality of life after pediatric intestinal transplantation: The perception of pediatric recipients and their parents. Am. J. Transplant. 2004, 4, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ngo, K.D.; Farmer, D.G.; McDiarmid, S.V.; Artavia, K.; Ament, M.E.; Vargas, J.; Busuttil, R.W.; Colangelo, J.; Esmailian, Y.; Gordon-Burroughs, S.; et al. Pediatric health-related quality of life after intestinal transplantation. Pediatr. Transplant. 2011, 15, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Folkman, S.; Lazarus, R.S.; Dunkel-Schetter, C.; DeLongis, A.; Gruen, R.J. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. J. Personal. Soc. Psychol. 1986, 50, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Essig, S.; von der Weid, N.X.; Strippoli, M.-P.F.; Rebholz, C.E.; Michel, G.; Rueegg, C.S.; Niggli, F.K.; Kuehni, C.E.; for the Swiss Pediatric Oncology Group (SPOG). Health-related quality of life in long-term survivors of relapsed childhood acute lymphoblastic leukemia. PLoS ONE 2012, 7, e38015. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.; Schwartz, C.E. The challenge of response shift for quality-of-life-based clinical oncology research. Ann. Oncol. 1999, 10, 747–749. [Google Scholar] [CrossRef]
- Olieman, J.F.; Penning, C.; Poley, M.J.; Utens, E.M.; Hop, W.C.; Tibboel, D. Impact of infantile short bowel syndrome on long-term health-related quality of life: A cross-sectional study. J. Pediatr. Surg. 2012, 47, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, F.; Khalil, B.; Morabito, A.; Wood, S.J. Impact of Short Bowel Syndrome on Quality of Life and Family: The Patient’s Perspective. Eur. J. Pediatr. Surg. 2019, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Yurtsever, S. Difficulties experienced by families with disabled children. J. Spéc. Pediatr. Nurs. 2007, 12, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Griffin, T. Between joy and sorrow: Being a parent of a child with developmental disability. J. Adv. Nurs. 2001, 34, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Yang, L.; Yu, Y.; Hou, J.; Li, J.; Li, Y.; Liu, H.; Zhang, Y.; Jiao, Z. Investigation of raising burden of children with autism, physical disability and mental disability in China. Res. Dev. Disabil. 2011, 32, 306–311. [Google Scholar] [CrossRef]
- Isa, S.N.I.; Ishak, I.; Ab Rahman, A.; Saat, N.Z.M.; Din, N.C.; Lubis, S.H.; Ismail, M.F.M. Health and quality of life among the caregivers of children with disabilities: A review of literature. Asian J. Psychiatry 2016, 23, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gottrand, F.; Staszewski, P.; Colomb, V.; Loras-Duclaux, I.; Guimber, D.; Marinier, E.; Breton, A.; Magnificat, S. Satisfaction in different life domains in children receiving home parenteral nutrition and their families. J. Pediatr. 2005, 146, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, A.; Maass, V.M.A. Entwicklungspsychologie des Kindes- und Jugendalters für Bachelor; Selbstkonzept: Berlin, Germany, 2010. [Google Scholar]
- Siegler, R.; Eisenberg, N.; DeLoache, J.; Saffran, J. Entwicklungs psychologie im Kindes und Jugendalter; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bisegger, C.; Cloetta, B.; von Bisegger, U.; Abel, T.; Ravens-Sieberer, U.; The European Kidscreen Group. Health-related quality of life: Gender differences in childhood and adolescence. Soz. Praventivmed. 2005, 50, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hommel, M.J.; van Baren, R.; Haveman, J.W. Surgical management and autologous intestinal reconstruction in short bowel syndrome. Best Pr. Res. Clin. Gastroenterol. 2016, 30, 263–280. [Google Scholar] [CrossRef]
- Nader, E.A.; Lambe, C.; Talbotec, C.; Pigneur, B.; Lacaille, F.; Garnier-Lengliné, H.; Petit, L.-M.; Poisson, C.; Rocha, A.; Corriol, O.; et al. Outcome of home parenteral nutrition in 251 children over a 14-y period: Report of a single center. Am. J. Clin. Nutr. 2016, 103, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, I.; Bjornestam, B.; Finkel, Y. Psychological distress associated with home parenteral nutrition in Swedish children, adolescents, and their parents: Preliminary results. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 246–250. [Google Scholar] [PubMed]
- Emedo, M.J.; Godfrey, E.I.; Hill, S.M. A qualitative study of the quality of life of children receiving intravenous nutrition at home. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 431–440. [Google Scholar] [CrossRef]
- Fullerton, B.S.; Hong, C.R.; Jaksic, T. Long-term outcomes of pediatric intestinal failure. Semin. Pediatr. Surg. 2017, 26, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Baglin-Gobet, S.; Talbotec, C.; Fourcade, L.; Colomb, V.; Sauvat, F.; Jais, J.-P.; Michel, J.-L.; Jan, D.; Ricour, C. Outcome and long-term growth after extensive small bowel resection in the neonatal period: A survey of 87 children. Eur. J. Pediatr. Surg. 2005, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Barros, G.G.; Tannuri, A.C.A.; Rotondo, Í.G.; Vaisberg, V.V.; Sarmento, L.S.; Neto, C.M.; Serafini, S.; de Oliveira Gonçalves, J.; Coelho, M.C.M.; Tannuri, U. Is maintenance of the ileocecal valve important to the intestinal adaptation mechanisms in a weaning rat model of short bowel? Pediatr. Surg. Int. 2018, 34, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Tejeira, R.E.; Ament, M.E.; Reyen, L.; Herzog, F.; Merjanian, M.; Olivares-Serrano, N.; Vargas, J.H. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: A 25-year experience. J. Pediatr. 2004, 145, 157–163. [Google Scholar] [CrossRef]
- Diamanti, A.; Basso, M.S.; Panetta, F.; Grimaldi, C.; Iacobelli, B.D.; Torre, G. Colon and intestinal adaptation in children with short bowel syndrome. J. Parenter. Enter. Nutr. 2012, 36, 501. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Colomb-Jung, V.; Joly, F. Role of the colon in short bowel syndrome and intestinal transplantation. J. Pediatr. Gastroenterol. Nutr. 2009, 48, S66–S71. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Randle, J. Living with a stoma: A review of the literature. J. Clin. Nurs. 2005, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kießling, C.; Wessel, L.M.; Felcht, J.; Hagl, C.I.; Boettcher, M.; Khasanov, R. Quality of Life of Children with Short Bowel Syndrome from Patients’ and Parents’ Points of View. Children 2024, 11, 536. https://doi.org/10.3390/children11050536
Kießling C, Wessel LM, Felcht J, Hagl CI, Boettcher M, Khasanov R. Quality of Life of Children with Short Bowel Syndrome from Patients’ and Parents’ Points of View. Children. 2024; 11(5):536. https://doi.org/10.3390/children11050536
Chicago/Turabian StyleKießling, Charlotte, Lucas M. Wessel, Judith Felcht, Cornelia I. Hagl, Michael Boettcher, and Rasul Khasanov. 2024. "Quality of Life of Children with Short Bowel Syndrome from Patients’ and Parents’ Points of View" Children 11, no. 5: 536. https://doi.org/10.3390/children11050536