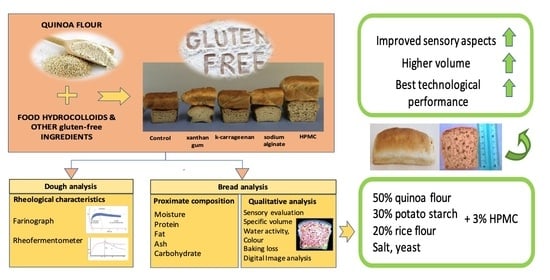
Effect of Different Hydrocolloids on the Qualitative Characteristics of Fermented Gluten-Free Quinoa Dough and Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characteristics of the Flours
2.3. Breadmaking Process
2.4. Dough Rheological Properties
2.5. Evaluation of Gluten-Free Breads
2.5.1. Proximate Composition, Specific Volume, Height, Water Activity, Color, and Baking Loss
2.5.2. Image Acquisition and Digital Image Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Chemical Composition of the Flours
3.2. Rheological Properties of Gluten-Free Doughs
3.3. Gluten-Free Bread Evaluation
Chemical Composition, Water Activity, Loaf Volume, Baking Loss, and Color
3.4. Digital Image Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mármol-Soler, C.; Matias, S.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.d.P.; Bustamante, M.Á.; Churruca, I.; Martínez, O.; Simón, E. Gluten-free products: Do we need to update our knowledge? Foods 2022, 11, 3839. [Google Scholar] [CrossRef] [PubMed]
- Capriles, V.D.; Valéria de Aguiar, E.; Garcia dos Santos, F.; Fernández, M.E.A.; de Melo, B.G.; Tagliapietra, B.L.; Scarton, M.; Clerici, M.T.P.S.; Conti, A.C. Current status and future prospects of sensory and consumer research approaches to gluten-free bakery and pasta products. Food Res. Int. 2023, 173, 113389. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. A systematic review of gluten-free dough and bread: Dough rheology, bread characteristics, and improvement strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Demirkesen, I.; Sumnu, G.; Sahin, S. Quality of gluten-free bread formulations baked in different ovens. Food Bioprocess Technol. 2013, 6, 746–753. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Novel approaches in gluten-free breadmaking: Interface between food science, nutrition, and health. Comp. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Bender, D.; Schönlechner, R. Innovative approaches towards improved gluten-free bread properties. J. Cereal Sci. 2020, 91, 102904. [Google Scholar] [CrossRef]
- Hager, A.S.; Arendt, E.K. Influence of hydroxypropylmethylcellulose (HPMC), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocoll. 2013, 32, 195–203. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Naik, H.R.; Zargar, I.A. Influence of hydrocolloids on dough handling and technological properties of gluten-free breads. Trends Food Sci. Technol. 2016, 51, 49–57. [Google Scholar] [CrossRef]
- Zoghi, A.; Mirmahdi, R.S.; Mohammadi, M. The role of hydrocolloids in the development of gluten-free cereal-based products for coeliac patients: A review. Int. J. Food Sci. Technol. 2021, 56, 3138–3147. [Google Scholar] [CrossRef]
- Sciarini, L.; Palavecino, P.M.; Ribotta, P.D. Use of additives in gluten-free formulations. In Designing Gluten Free Bakery and Pasta Products; Springer International Publishing: Cham, Switzerland, 2023; pp. 115–161. [Google Scholar]
- Salehi, F. Improvement of gluten-free bread and cake properties using natural hydrocolloids: A review. Food Sci. Nutr. 2019, 7, 3391–3402. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallangher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Thomas Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Axel, C.; Rocker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorous DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavouring production in kamut, quinoa and wheat doughs fermented by Lactobacillus paracasei, Lactobacillus plantarum and Lactobacillus brevis: A SPME-GC/MS study. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Messia, M.C.; Pulvento, C.; Lavini, A.; Nazzaro, S.; Di Renzo, T. Microbial and qualitative traits of quinoa and amaranth seeds from experimental fields in southern Italy. Foods 2023, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- International Association for Cereal Science and Technology. Standard Methods of the International Association for Cereal Science and Technology; ICC: Wien, Austria, 1995. [Google Scholar]
- AACC. Approved Methods of the American Association of Cereal Chemists, Volume 1; American Association of Cereal Chemists Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Cereal Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, F.; Huang, W.; Zheng, J.; Rayas-duarte, P. Rheofermentometer fermentation and breadmaking characteristics of dough containing xylo-oligosaccharide hydrolyzate from wheat bran. J. Agric. Food Chem. 2010, 58, 1878–1883. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallangher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2010, 230, 437–445. [Google Scholar] [CrossRef]
- Mariotti, M.; Pagani, M.A.; Lucisano, M. The role of buckwheat and HPMC on the breadmaking properties of some commercial gluten-free bread mixtures. Food Hydrocoll. 2013, 30, 393–400. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Preziuso, M.; Panfili, G.; Cipriano, L.; Messia, M.C. Stabilization of sourdough starter by spray drying technique: New breadmaking perspective. LWT—Food Sci. Technol. 2019, 99, 468–475. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Alamri, M.S.; Hussain, S.; Ibraheem, M.A.; Qasem, A.A.A.; Shamlan, G.; Ababtain, I.A. Effect of ziziphus and cordia gums on dough properties and baking performance of cookies. Molecules 2022, 27, 3066. [Google Scholar] [CrossRef] [PubMed]
- Morreale, F.; Garzón, R.; Rosell, C.M. Understanding the role of hydrocolloids viscosity and hydration in developing gluten-free bread. A study with hydroxypropylmethylcellulose. Food Hydrocoll. 2018, 77, 629–635. [Google Scholar] [CrossRef]
- Imamura, M.; Seiko, I.; Arai, E. Effects of different types of hydrocolloids on the quality improvement of gluten-free rice flour bread made with soymilk. Food Sci. Technol. Res. 2021, 27, 389–395. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Yamul, K.D.; Sun, H.; Zhang, M.; Chen, J.; Fauconnier, M.L.; Andrea, P.V. Evaluation of different hydrocolloids to improve dough rheological properties and bread quality of potato–wheat flour. J. Food Sci. Technol. 2017, 54, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M.; Rojas, J.A.; Benedito de Barber, C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Armero, E.; Collar, C. Antistaling additive effects on fresh wheat bread quality. Food Sci. Technol. Int. 1996, 2, 323–333. [Google Scholar]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M.J. Differential hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- Davidou, S.; Le Mestre, M.; Debever, E.; Bekaert, D. A contribution to the study of staling of white bread: Effect of water and hydrocolloid. Food Hydrocoll. 1996, 10, 375–383. [Google Scholar] [CrossRef]
- Milani, J.M.; Maleki, G. Hydrocolloids in food industry. In Food Industrial Processes—Methods and Equipment; Valdez, B., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 17–38. [Google Scholar]
- Nammakuna, N.; Barringer, S.A.; Ratanatriwong, P. The effects of protein isolates and hydrocolloids complexes on dough rheology, physicochemical properties and qualities of gluten-free crackers. Food Sci. Nutr. 2016, 4, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Marti, A.; Marengo, M.; Bonomi, F.; Casiraghi, M.C.; Franzetti, L.; Pagani, M.A.; Iametti, S. Molecular features of fermented teff flour relate to its suitability for the production of enriched gluten-free bread. LWT—Food Sci. Technol. 2017, 78, 296–302. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Gu, Z.; Li, Z. Co-supported hydrocolloids improve the structure and texture quality of gluten-free bread. LWT—Food Sci. Technol. 2021, 152, 112248. [Google Scholar] [CrossRef]
- Franco, W.; Evert, K.; Van Nieuwenhove, C. Quinoa flour, the germinated grain flour, and sourdough as alternative sources for gluten-free bread formulation: Impact on chemical, textural and Sensorial Characteristics. Fermentation 2021, 7, 115. [Google Scholar] [CrossRef]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Ribotta, P.D.; León, A.E.; Pérez, G.T. Effect of hydrocolloids on gluten-free batter properties and bread quality. Int. J. Food Sci. Technol. 2010, 45, 2306–2312. [Google Scholar] [CrossRef]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Gidley, M.J.; Caswell, D.C. Thermogelation of methylcellulose. Part II: Effect of hydroxypropyl substituents. Carbohydr. Polym. 1993, 22, 175–186. [Google Scholar] [CrossRef]
- Houben, A.; Höchstötter, A.; Becker, T. Possibilities to increase the quality in gluten-free bread production: An overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar] [CrossRef]



| Ingredients | Sample Code | ||||
|---|---|---|---|---|---|
| Control | d-KC | d-XG | d-SA | d-HPMC | |
| Quinoa flour | 50 | 50 | 50 | 50 | 50 |
| Rice flour | 20 | 20 | 20 | 20 | 20 |
| Potato starch | 30 | 30 | 30 | 30 | 30 |
| Salt | 1.75 | 1.75 | 1.75 | 1.75 | 1.75 |
| Sugar | 1 | 1 | 1 | 1 | 1 |
| Yeast | 2 | 2 | 2 | 2 | 2 |
| κ-carrageenan (KC) | 3 | ||||
| Xanthan gum (XG) | 3 | ||||
| Sodium alginate (SA) | 3 | ||||
| Hydroxypropylmethylcellulose (HPMC) | 3 | ||||
| Flour Samples | Protein | Fat | Ash | Fiber | Carbohydrates * |
|---|---|---|---|---|---|
| Q | 15.6 ± 0.16 a | 6.1 ± 0.10 a | 5.59 ± 0.12 a | 12.1 ± 1.02 a | 60.8 ± 0.50 d |
| R | 8.3 ± 0.11 c | 0.6 ± 0.05 c | 0.71 ± 0.01 c | 1.14 ± 0.05 c | 89.3 ± 0.21 b |
| P | 1.1 ± 0.20 d | 0.01 ± 0.01 d | 0.35 ± 0.01 d | 0.01 ± 0.10 d | 98.5 ± 0.21 a |
| QRP | 10.1 ± 0.15 b | 3.1 ± 0.02 b | 3.04 ± 0.12 b | 6.3 ± 0.05 b | 77.5 ± 0.15 c |
| Sample | ||||
|---|---|---|---|---|
| d-KC | d-SA | d-XG | d-HPMC | |
| Farinographic index | ||||
| Water absorption (%) | 57.5 b | 54.0 c | 60.4 a | 59.2 a |
| Dough development time DDT(min) | 4.5 d | 20 a | 14.7 b | 9.1 c |
| Stability (min) | 1 c | 1.5 c | 7.3 b | 13 a |
| Degree of softening (FU) | 95 b | 164 a | 74 c | 5 d |
| Farinograph quality number (FQN) | 55 c | 200 a | 147 b | 200 a |
| Rheofermentographic index | ||||
| Hm (mm) | 13.3 b | 12.7 b | 8.9 c | 21.2 a |
| T1 (min) | 177 b | 178 b | 72 c | 180 a |
| Tx (min) | 58 b | 79 a | 61 b | 81 a |
| Total volume Vt (mL) | 1127 b | 1051 d | 1193 a | 1103 c |
| Retention coefficient Vr (%) | 88.1 c | 86.9 d | 90.7 b | 93 a |
| Samples | Moisture (%) | Protein | Fat | Ash | Starch * | Water Activity (aw) |
|---|---|---|---|---|---|---|
| (% d.w.) | (% d.w.) | (% d.w.) | (% d.w.) | |||
| b-Control | 38.7 ± 0.26 d | 9.8 ± 0.04 c | 2.7 ± 0.03 a | 2.97 ± 0.078 d | 70.2 ± 0.15 a | 0.96 ± 0.004 c |
| b-KC | 43.8 ± 0.01 c | 10.3 ± 0.01 a | 2.9 ± 0.14 a | 3.35 ± 0.021 b | 67.6 ± 0.21 b | 0.97 ± 0.002 b |
| b-XG | 46.2 ± 0.00 b | 9.9 ± 0.03 b | 2.7 ± 0.08 a | 3.20 ± 0.035 c | 67.2 ± 0.13 b | 0.99 ± 0.001 a |
| b-SA | 46.1 ± 0.10 b | 10.1 ± 0.17 b | 2.7 ± 0.04 a | 4.07 ± 0.064 a | 65.6 ± 0.04 c | 0.97 ± 0.005 b |
| b-HPMC | 50.8 ± 0.34 a | 9.7 ± 0.15 c | 2.8 ± 0.12 a | 3.01 ± 0.035 d | 67.9 ± 0.21 b | 0.98 ± 0.007 b |
| Bread Sample | Specific Volume (mL/g) | Bake Loss (%) | Height (cm) | Color | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crust | Crumb | ||||||||||
| L* | a* | b* | ΔE* | L* | a* | b* | ΔE* | ||||
| b-Control | 1.59 ± 0.03 b | 11.13 ± 0.15 b | 3.83 ± 0.06 c | 37.5 ± 2.26 a | 14.0 ± 0.44 a | 28.3 ± 2.10 a | - | 48.8 ± 1.36 b | 1.0 ± 0.35 b | 18.9 ± 0.95 a | - |
| b-KC | 1.61 ± 0.05 b | 10.64 ± 0.44 b | 3.93 ± 0.06 c | 40.8 ± 1.00 a | 12.0 ± 0.79 a | 29.8 ± 1.14 a | 4.80 ± 1.82 a | 41.3 ± 5.78 a | 0.8 ± 0.41 b | 20.1 ± 0.64 b,a | 8.60 ± 4.97 a |
| b-XG | 1.29 ± 0.07 a | 7.98 ± 1.11 a | 5.20 ± 0.2 b | 41.8 ± 2.48 a | 13.6 ± 0.35 a | 32.5 ± 1.04 b | 6.90 ± 3.84 a | 45.3 ± 2.92 b,a | 0.9 ± 0.33 b | 20.8 ± 0.54 b,a | 5.00 ± 2.16 a |
| b-SA | 1.70 ± 0.02 c | 10.30 ± 0.31 b | 4.90 ± 0.1 b | 39.8 ± 6.50 a | 13.0 ± 2.09 a | 30.9 ± 2.43 a | 7.07 ± 3.88 a | 58.5 ± 0.69 c | 0.3 ± 0.11 a | 18.4 ± 0.33 a | 8.80 ± 1.38 a,b |
| b-HPMC | 2.29 ± 0.10 d | 7.38 ± 0.43 a | 6.77 ± 0.06 a | 36.0 ± 6.91 a | 11.9 ± 0.56 a | 29.2 ± 1.02 a | 8.30 ± 2.09 a | 35.8 ± 3.89 a | 0.8 ± 0.33 b | 18.1 ± 0.69 a | 14.10 ± 4.81 b,a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, T.; Trivisonno, M.C.; Nazzaro, S.; Reale, A.; Messia, M.C. Effect of Different Hydrocolloids on the Qualitative Characteristics of Fermented Gluten-Free Quinoa Dough and Bread. Foods 2024, 13, 1382. https://doi.org/10.3390/foods13091382
Di Renzo T, Trivisonno MC, Nazzaro S, Reale A, Messia MC. Effect of Different Hydrocolloids on the Qualitative Characteristics of Fermented Gluten-Free Quinoa Dough and Bread. Foods. 2024; 13(9):1382. https://doi.org/10.3390/foods13091382
Chicago/Turabian StyleDi Renzo, Tiziana, Maria Carmela Trivisonno, Stefania Nazzaro, Anna Reale, and Maria Cristina Messia. 2024. "Effect of Different Hydrocolloids on the Qualitative Characteristics of Fermented Gluten-Free Quinoa Dough and Bread" Foods 13, no. 9: 1382. https://doi.org/10.3390/foods13091382