Gel Properties and Protein Structures of Minced Pork Prepared with κ-Carrageenan and Non-Meat Proteins
Abstract
:1. Introduction
2. Results and Discussion
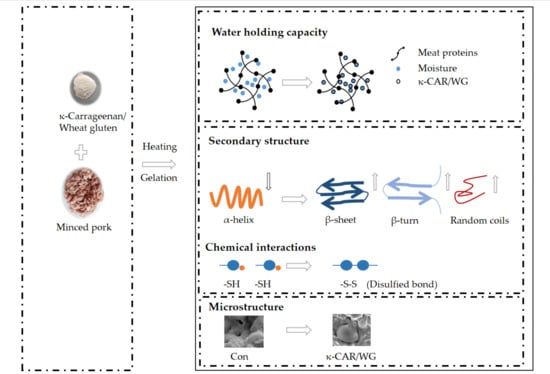
2.1. Cooking Loss and WHC
2.2. Color
2.3. Texture Profile Analysis
2.4. Protein Secondary Structure
2.5. DSC
2.6. Analysis of Chemical Interactions
2.7. Scanning Electron Microscopy
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Minced Pork
4.3. Cooking Loss (CL)
4.4. Water-Holding Capacity (WHC)
4.5. Color
4.6. Texture Profile Analysis (TPA)
4.7. Secondary Structure via Raman Spectroscopy
4.8. Thermal Stability Analysis
4.9. Determination of Intermolecular Forces
4.10. Scanning Electron Microscopy (SEM)
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Zhan, W.; Du, Z.; Hong, S.; Dong, T.; She, J.; Min, C. Pork Primal Cuts Recognition Method via Computer Vision. Meat Sci. 2022, 192, 108898. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, L.; Zareef, M.; Wang, L.; Chen, Q. Application of Portable Visible and Near-Infrared Spectroscopy for Rapid Detection of Cooking Loss Rate in Pork: Comparing Spectra from Frozen and Thawed Pork. LWT 2022, 160, 113304. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhou, G. Covalent Chemical Modification of Myofibrillar Proteins to Improve Their Gelation Properties: A Systematic Review. Comp. Rev. Food Sci. Food Saf. 2021, 20, 924–959. [Google Scholar] [CrossRef]
- Chen, J.; Deng, T.; Wang, C.; Mi, H.; Yi, S.; Li, X.; Li, J. Effect of Hydrocolloids on Gel Properties and Protein Secondary Structure of Silver Carp Surimi. J. Sci. Food Agric. 2020, 100, 2252–2260. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, C.; Xu, X.; Zeng, X.; Xing, T. Ultrasound Combined with Carrageenan and Curdlan Addition Improved the Gelation Properties of Low-Salt Chicken Meat Paste. LWT 2022, 172, 114230. [Google Scholar] [CrossRef]
- Qiao, M.; Zhang, T.; Miao, M. Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid. Foods 2024, 13, 510. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, S.; Jia, X.; Wang, H.; Zhang, H.; Liu, Q.; Kong, B. Thermal Gelling Properties and Structural Properties of Myofibrillar Protein Including Thermo-Reversible and Thermo-Irreversible Curdlan Gels. Food Chem. 2020, 311, 126018. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.-Y.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Research Progress on Polysaccharide/Protein Hydrogels: Preparation Method, Functional Property and Application as Delivery Systems for Bioactive Ingredients. Food Res. Int. 2021, 147, 110542. [Google Scholar] [CrossRef]
- Kim, T.-K.; Shim, J.-Y.; Hwang, K.-E.; Kim, Y.-B.; Sung, J.-M.; Paik, H.-D.; Choi, Y.-S. Effect of Hydrocolloids on the Quality of Restructured Hams with Duck Skin. Poult. Sci. 2018, 97, 4442–4449. [Google Scholar] [CrossRef]
- Li, S.; Lin, S.; Jiang, P.; Bao, Z.; He, X.; Sun, N. Contribution of κ-/ι-carrageenan on the Gelling Properties of Shrimp Myofibrillar Protein and Their Interaction Mechanism Exploration. J. Sci. Food Agric. 2023, 103, 524–533. [Google Scholar] [CrossRef]
- Zheng, L.; Regenstein, J.M.; Zhou, L.; Mokhtar, S.M.; Wang, Z. Gel Properties and Structural Characteristics of Composite Gels of Soy Protein Isolate and Silver Carp Protein. Gels 2023, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Xu, J.; Ji, F.; Tan, M.; Luo, S.; Zhong, X.; Zheng, Z. Properties of Transglutaminase-induced Myofibrillar/Wheat Gluten Gels. J. Food Sci. 2021, 86, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, Y.; Han, J.; Liu, Q.; Kong, B. Gelation and Rheological Properties of Myofibrillar Proteins Influenced by the Addition of Soybean Protein Isolates Subjected to an Acidic pH Treatment Combined with a Mild Heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Jin, S.; Kim, S.-H.; Choi, J.-S.; Yim, D.-G. Effect of Diverse Binder Materials and Their Addition Levels on Physico-Chemical Characteristics of Sausages. Food Meas. 2019, 13, 1558–1565. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Zhong, X.; Cai, K.; Cai, J.; Jiang, S.; Zheng, Z. Effect of Modified Wheat Gluten on Boiling Resistance Capacity of Pork Meatballs. J. Food Sci. 2016, 81, E430–E437. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Feng, Y.; Kong, B.; Xia, X.; Liu, M.; Chen, J.; Zhang, F.; Liu, Q. Textural and Gel Properties of Frankfurters as Influenced by Various κ-Carrageenan Incorporation Methods. Meat Sci. 2021, 176, 108483. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiong, Y.L. Interaction of Myofibrillar and Preheated Soy Proteins. J. Food Sci. 2002, 67, 2851–2856. [Google Scholar] [CrossRef]
- Petruccelli, S.; Anon, M.C. Relationship between the Method of Obtention and the Structural and Functional Properties of Soy Proteins Isolates. 1. Structural and Hydration Properties. J. Agric. Food Chem. 1994, 42, 2161–2169. [Google Scholar] [CrossRef]
- Sun, J.; Yin, G.; Chen, J.; Li, P. Gelling Properties of Myofibrillar Protein–Soy Protein and k-Carrageenan Composite as Affected by Various Salt Levels. Int. J. Food Prop. 2019, 22, 2047–2056. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.; Zhou, G. Emulsion Stability, Thermo-rheology and Quality Characteristics of Ground Pork Patties Prepared with Soy Protein Isolate and Carrageenan. J. Sci. Food Agric. 2015, 95, 2832–2837. [Google Scholar] [CrossRef]
- Wasinnitiwong, N.; Benjakul, S.; Hong, H. Effects of κ-Carrageenan on Gel Quality of Threadfin Bream (Nemipterus Spp.) Surimi Containing Salted Duck Egg White Powder. Int. J. Biol. Macromol. 2022, 221, 61–70. [Google Scholar] [CrossRef]
- Álvarez, D.; Xiong, Y.L.; Castillo, M.; Payne, F.A.; Garrido, M.D. Textural and Viscoelastic Properties of Pork Frankfurters Containing Canola–Olive Oils, Rice Bran, and Walnut. Meat Sci. 2012, 92, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.-L.; Chen, F.-S.; Ma, H.-J. Effect of Pre-Emulsified Soy Oil with Soy Protein Isolate in Frankfurters: A Physical-Chemical and Raman Spectroscopy Study. LWT 2016, 74, 465–471. [Google Scholar] [CrossRef]
- Shin, D.-M.; Yune, J.H.; Kim, Y.J.; Keum, S.H.; Jung, H.S.; Kwon, H.C.; Kim, D.H.; Sohn, H.; Jeong, C.H.; Lee, H.G.; et al. Effects of Duck Fat and κ-Carrageenan as Replacements for Beef Fat and Pork Backfat in Frankfurters. Anim. Biosci. 2022, 35, 927–937. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, J.; Xue, Y.; Xue, C. Enhancing Gel Performance of Surimi Gels via Emulsion Co-Stabilized with Soy Protein Isolate and κ-Carrageenan. Food Hydrocoll. 2023, 135, 108217. [Google Scholar] [CrossRef]
- Zhuang, X.; Jiang, X.; Zhou, H.; Chen, Y.; Zhao, Y.; Yang, H.; Zhou, G. Insight into the Mechanism of Physicochemical Influence by Three Polysaccharides on Myofibrillar Protein Gelation. Carbohydr. Polym. 2020, 229, 115449. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, S.; Laskar, S.K.; Das, A.; Upadhyay, S.; Hazarika, R.A.; Mahanta, J.D.; Sangtam, H.M. Effect of Incorporation of Soy Protein Isolate and Inulin on Quality Characteristics and Shelf-Life of Low–Fat Duck Meat Sausages. Anim. Biosci. 2022, 35, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zang, M.; Wang, S.; Zhao, B.; Xu, C.; Bai, J.; Zhao, Y.; Qiao, X.; Wu, J. Effects of Citrus Fibre and Soybean Protein Isolate on Heat-induced Pork Myofibrillar Protein Gel Properties under Low-sodium Salt Conditions. Int. J. Food Sci. Technol. 2022, 57, 7701–7711. [Google Scholar] [CrossRef]
- Wang, K.-Q.; Luo, S.-Z.; Zhong, X.-Y.; Cai, J.; Jiang, S.-T.; Zheng, Z. Changes in Chemical Interactions and Protein Conformation during Heat-Induced Wheat Gluten Gel Formation. Food Chem. 2017, 214, 393–399. [Google Scholar] [CrossRef]
- Yi, S.; Wu, Q.; Tong, S.; Wang, W.; Li, X.; Mi, H.; Xu, Y.; Li, J. Thermal Aggregation Behavior of Egg White Protein and Blue Round Scad ( Decapterus Maruadsi ) Myofibrillar Protein. J. Food Sci. 2022, 87, 3900–3912. [Google Scholar] [CrossRef]
- Bak, K.H.; Bolumar, T.; Karlsson, A.H.; Lindahl, G.; Orlien, V. Effect of High Pressure Treatment on the Color of Fresh and Processed Meats: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S. Impact of Salted Duck Egg Albumen Powder on Proteolysis and Gelling Properties of Sardine Surimi. J. Texture Stud. 2019, 50, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tan, L.; Chen, Y.; Zhang, J.; Li, H.; Chen, L. Effect of Κ-carrageenan Addition on Protein Structure and Gel Properties of Salted Duck Egg White. J. Sci. Food Agric. 2021, 101, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Wang, P.; Zou, M.; Gu, Z.; Yang, R. Heat-Induced Polymerization Behavior Variation of Frozen-Stored Gluten. Food Chem. 2018, 255, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Wang, Y.; Wang, R.; Zeng, M. Effect of κ-Carrageenan on the Gelation Properties of Oyster Protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, Q.; He, N.; Wang, Q. High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality. Foods 2022, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-Y.; Li, Y.-Q. The Role of Secondary Structure in Protein Structure Selection. Eur. Phys. J. E 2010, 32, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kinalwa, M.N.; Blanch, E.W.; Doig, A.J. Accurate Determination of Protein Secondary Structure Content from Raman and Raman Optical Activity Spectra. Anal. Chem. 2010, 82, 6347–6349. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, W.; Li, X.; Yi, S.; Mi, H.; Xu, Y.; Li, J. Gel Properties of Blue Round Scad (Decapterus maruadsi) Mince as Influenced by the Addition of Egg White Powder. J. Texture Stud. 2022, 53, 563–576. [Google Scholar] [CrossRef]
- Eyiler Yilmaz, E.; Vural, H.; Jafarzadeh Yadigari, R. Thermal, Microscopic, and Quality Properties of Low-Fat Frankfurters and Emulsions Produced by Addition of Different Hydrocolloids. Int. J. Food Prop. 2017, 20, 1987–2002. [Google Scholar] [CrossRef]
- Debusca, A.; Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Physicochemical Properties of Surimi Gels Fortified with Dietary Fiber. Food Chem. 2014, 148, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, J.; Zheng, J.; Li, X.; Shao, J.-H. The Study of Protein Conformation and Hydration Characteristics of Meat Batters at Various Phase Transition Temperatures Combined with Low-Field Nuclear Magnetic Resonance and Fourier Transform Infrared Spectroscopy. Food Chem. 2019, 280, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, T.-K.; Ku, S.-K.; Kim, M.J.; Jung, S.; Yong, H.I.; Choi, Y.-S. Quality Characteristics of Semi-Dried Restructured Jerky: Combined Effects of Duck Skin Gelatin and Carrageenan. J. Anim. Sci. Technol. 2020, 62, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The Interaction of Starch-Gums and Their Effect on Gel Properties and Protein Conformation of Silver Carp Surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Sukmanov, V.; Ma, H. Effects of Soy Protein Isolate on Gel Properties and Water Holding Capacity of Low-Salt Pork Myofibrillar Protein under High Pressure Processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-L.; Han, M.-Y.; Fei, Y.; Zhou, G.-H. Raman Spectroscopic Study of Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Textural Characteristic. Meat Sci. 2011, 87, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Alix, A.J.P.; Pedanou, G.; Berjot, M. Fast Determination of the Quantitative Secondary Structure of Proteins by Using Some Parameters of the Raman Amide I Band. J. Mol. Struct. 1988, 174, 159–164. [Google Scholar] [CrossRef]
- Tan, F.; Lai, K.; Hsu, K. A comparative study on physical properties and chemical interactions of gels from tilapia meat pastes induced by heat and pressure. J. Texture Stud. 2010, 41, 153–170. [Google Scholar] [CrossRef]



| Treatment 1 | Cooking Loss (%) | WHC 2 (%) | L* | a* | b* |
|---|---|---|---|---|---|
| Con | 17.26 ± 0.22 a | 75.25 ± 0.14 g | 74.90 ± 0.06 a | 1.10 ± 0.03 d | 11.68 ± 0.09 b |
| κ-CAR | 16.73 ± 0.05 b | 77.95 ± 0.28 f | 74.61 ± 0.44 a | 1.60 ± 0.10 abc | 13.55 ± 0.20 a |
| WG | 10.92 ± 0.10 d | 78.77 ± 0.47 e | 74.07 ± 0.50 a | 1.91 ± 0.22 a | 13.10 ± 0.23 a |
| EWP | 10.62 ± 0.23 d | 79.95 ± 0.11 d | 75.03 ± 0.24 a | 1.62 ± 0.14 ab | 12.99 ± 0.73 a |
| SPI | 1.31 ± 0.01 e | 91.42 ± 0.20 a | 72.51 ± 0.35 b | 1.28 ± 0.16 bcd | 13.07 ± 0.44 a |
| C-E | 16.18 ± 0.30 c | 77.53 ± 0.22 f | 74.53 ± 0.05 a | 1.22 ± 0.05 cd | 12.53 ± 0.23 b |
| C-W | 10.98 ± 0.16 d | 81.91 ± 0.07 c | 74.50 ± 0.19 a | 1.40 ± 0.03 bcd | 12.52 ± 0.38 b |
| C-S | 0.98 ± 0.14 e | 87.37 ± 0.23 b | 74.53 ± 0.05 a | 1.22 ± 0.05 cd | 12.52 ± 0.23 b |
| Treatment 1 | Hardness (N) | Cohesiveness | Springiness (%) | Chewiness (N) |
|---|---|---|---|---|
| Con | 20.61 ± 1.21 f | 0.38 ± 0.01 b | 0.82 ± 0.01 c | 6.35 ± 7.94 e |
| κ-CAR | 19.86 ± 0.89 g | 0.34 ± 0.01 c | 0.74 ± 0.01 d | 4.90 ± 0.47 f |
| EWP | 25.82 ± 0.91 c | 0.41 ± 0.01 a | 0.83 ± 0.01 c | 8.79 ± 0.58 c |
| WG | 38.91 ± 0.80 a | 0.43 ± 0.01 a | 0.88 ± 0.01 a | 14.73 ± 0.41 a |
| SPI | 22.56 ± 0.23 d | 0.31 ± 0.01 d | 0.66 ± 0.01 e | 4.56 ± 0.03 g |
| C-E | 21.61 ± 0.29 e | 0.39 ± 0.01 b | 0.82 ± 0.01 c | 6.86 ± 0.27 d |
| C-W | 27.93 ± 0.38 b | 0.42 ± 0.01 a | 0.85 ± 0.01 b | 9.83 ± 0.36 b |
| C-S | 20.59 ± 0.24 f | 0.24 ± 0.01 e | 0.57 ± 0.02 f | 2.82 ± 0.15 h |
| Treatment 1 | Td (°C) | ∆H (J/g) |
|---|---|---|
| Con | 66.70 ± 0.12 a | 0.33 ± 0.04 g |
| κ-CAR | 63.50 ± 0.06 d | 0.39 ± 0.02 f |
| EWP | 65.00 ± 0.17 b | 0.45 ± 0.00 e |
| WG | 63.40 ± 0.23 d | 0.68 ± 0.02 b |
| SPI | 64.00 ± 0.29 cd | 0.57 ± 0.02 c |
| C-E | 65.43 ± 0.09 b | 0.51 ± 0.01 d |
| C-W | 64.00 ± 0.23 b | 0.75 ± 0.01 a |
| C-S | 64.30 ± 0.25 cd | 0.51 ± 0.03 d |
| Treatment 1 | Amount of Each Substance Added to Minced Meat 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Meat | Backfat | κ-CAR | EWP | WG | SPI | Salt | Ice Water | |
| Con | 80 | 20 | - | - | - | - | 2 | 20 |
| κ-CAR | 80 | 20 | 0.5 | - | - | - | 2 | 20 |
| WG | 80 | 20 | - | 4 | - | - | 2 | 20 |
| EWP | 80 | 20 | - | - | 6 | - | 2 | 20 |
| SPI | 80 | 20 | - | - | - | 5 | 2 | 20 |
| C-E | 80 | 20 | 0.5 | 4 | - | - | 2 | 20 |
| C-W | 80 | 20 | 0.5 | - | 6 | - | 2 | 20 |
| C-S | 80 | 20 | 0.5 | - | - | 5 | 2 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Chen, F.; Shi, M.; Wang, Y.; Xiao, X.; Wu, C. Gel Properties and Protein Structures of Minced Pork Prepared with κ-Carrageenan and Non-Meat Proteins. Gels 2024, 10, 305. https://doi.org/10.3390/gels10050305
Ye Y, Chen F, Shi M, Wang Y, Xiao X, Wu C. Gel Properties and Protein Structures of Minced Pork Prepared with κ-Carrageenan and Non-Meat Proteins. Gels. 2024; 10(5):305. https://doi.org/10.3390/gels10050305
Chicago/Turabian StyleYe, Yang, Fei Chen, Meimei Shi, Yang Wang, Xia Xiao, and Chunmei Wu. 2024. "Gel Properties and Protein Structures of Minced Pork Prepared with κ-Carrageenan and Non-Meat Proteins" Gels 10, no. 5: 305. https://doi.org/10.3390/gels10050305