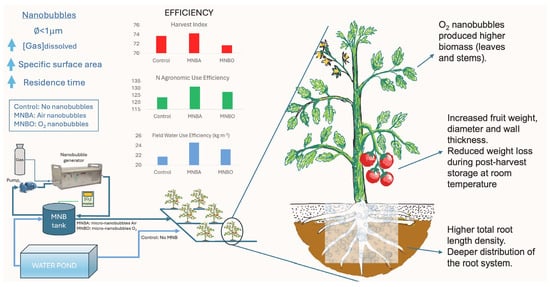
Enhancing Root Distribution, Nitrogen, and Water Use Efficiency in Greenhouse Tomato Crops Using Nanobubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Crop
2.3. Experimental Design
2.3.1. Experimental Treatments
2.3.2. Generation of Nanobubbles
2.4. Soil Measurements
2.4.1. Soil Sampling
2.4.2. Soil Analyses
2.4.3. Soil Pore Water Sampling
2.5. Crop Measurements
2.5.1. Root Sampling
2.5.2. Petiole Sap Nutrient Status
2.5.3. Above-Ground Dry Matter (Leaves and Stems) Production
2.5.4. Fruit Production and Quality
2.5.5. Weight Lost during Postharvest Storage
2.5.6. Indices
2.6. Statistical Analysis
3. Results
3.1. Soil Measurements
3.1.1. Soil
3.1.2. Soil Pore Water
3.2. Crop Measurements
3.2.1. Root Length Density
3.2.2. Petiole Sap
3.2.3. Above-Ground Fresh and Dry Matter (Leaves and Stems) Production
3.2.4. Fruit Production and Quality
3.2.5. Weight Lost during Postharvest Storage
3.2.6. Indices
4. Discussion
4.1. Soil Measurements
4.2. Crop Measurements
4.2.1. Root Length Density and Petiole Sap
4.2.2. Above-Ground Fresh and Dry Matter (Leaves and Stems) Production
4.2.3. Fruit Production, Quality, and Weight Lost during Postharvest Storage
4.3. Recommendations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Noah, I.; Nitsan, I.; Cohen, B.; Kaplan, G.; Friedman, S.P. Soil Aeration Using Air Injection in a Citrus Orchard with Shallow Groundwater. Agric. Water Manag. 2021, 245, 106664. [Google Scholar] [CrossRef]
- Pineda Pineda, J.; Moreno Roblero, M.d.J.; Colinas León, M.T.; Sahagún Castellanos, J. El Oxígeno En La Zona Radical y Su Efecto En Las Plantas. Rev. Mex. Cienc. Agric. 2020, 11, 931–943. [Google Scholar] [CrossRef]
- Carazo, N. Oxifertirrigación En Cultivo Sin Suelo de Rosa Para Flor Cortada (Rosa Sp.) y Pimiento (Capsicum annuum L.): Efectos En Desarrollo y Producción. Ph.D. Thesis, Universidad de Lleida, Lleida, Spain, 2015. [Google Scholar]
- Bhattarai, S.P.; Su, N.; Midmore, D.J. Oxygation Unlocks Yield Potentials of Crops in Oxygen-Limited Soil Environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar] [CrossRef]
- Jin, C.; Lei, H.; Chen, J.; Xiao, Z.; Leghari, S.J.; Yuan, T.; Pan, H. Effect of Soil Aeration and Root Morphology on Yield under Aerated Irrigation. Agronomy 2023, 13, 369. [Google Scholar] [CrossRef]
- Dresbøll, D.B.; Thorup-Kristensen, K. Spatial Variation in Root System Activity of Tomato (Solanum lycopersicum L.) in Response to Short and Long-Term Waterlogging as Determined by 15N Uptake. Plant Soil. 2012, 357, 161–172. [Google Scholar] [CrossRef]
- Ben-Noah, I.; Friedman, S.P. Review and Evaluation of Root Respiration and of Natural and Agricultural Processes of Soil Aeration. Vadose Zone J. 2018, 17, 1–47. [Google Scholar] [CrossRef]
- Abuarab, M.; Mostafa, E.; Ibrahim, M. Effect of Air Injection under Subsurface Drip Irrigation on Yield and Water Use Efficiency of Corn in a Sandy Clay Loam Soil. J. Adv. Res. 2013, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.P.; Pendergast, L.; Midmore, D.J. Root Aeration Improves Yield and Water Use Efficiency of Tomato in Heavy Clay and Saline Soils. Sci. Hortic. 2006, 108, 278–288. [Google Scholar] [CrossRef]
- Vyrlas, P.; Sakellariou-Makrantonaki, M.; Kalfountzos, D. Aerogation: Crop Root-Zone Aeration through Subsurface Drip Irrigation System. WSEAS Trans. Environ. Dev. 2014, 10, 250–255. [Google Scholar]
- Pendergast, L.; Bhattarai, S.P.; Midmore, D.J. Evaluation of Aerated Subsurface Drip Irrigation on Yield, Dry Weight Partitioning and Water Use Efficiency of a Broad-Acre Chickpea (Cicer arietinum, L.) in a Vertosol. Agric. Water Manag. 2019, 217, 38–46. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, J.; Jiang, Y.; Hu, D.; Yang, X.; Dong, M.; Yu, K.; Yu, S. Effect of Rhizosphere Aeration by Subsurface Drip Irrigation with Tanks on the Growth of ‘Red Globe’ Grape Seedling and Its Absorption, Distribution and Utilization of Urea- 15 N. Sci. Hortic. 2018, 236, 207–213. [Google Scholar] [CrossRef]
- Park, J.S.; Ohashi, K.; Kurata, K.; Lee, J.W. Promotion of Lettuce Growth by Application of Microbubbles in Nutrient Solution Using Different Rates of Electrical Conductivity and under Periodic Intermittent Generation in a Deep Flow Technique Culture System. Eur. J. Hortic. Sci. 2010, 75, 198–203. [Google Scholar]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative Cultivation and Biochemical Analysis of Iceberg Lettuce Grown in Sand Soil and Hydroponics With or Without Microbubbles and Macrobubbles. J. Soil. Sci. Plant Nutr. 2021, 21, 389–403. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.M.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Mazuela, P.C. Effect of Slow-Release Oxygen Supply by Fertigation on Horticultural Crops under Soilless Culture. Sci. Hortic. 2005, 106, 484–490. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, D.; Zhou, J.; Xu, Z.; Mou, P.; Cao, Y.; Zhu, J. Oxygenated Compound Fertilizer Improved Rice Yield by Empowering Soil Aeration. Commun. Soil. Sci. Plant Anal. 2021, 52, 1798–1810. [Google Scholar] [CrossRef]
- Wang, R.; Shi, W.; Kronzucker, H.J.; Li, Y. Oxygenation Promotes Vegetable Growth by Enhancing P Nutrient Availability and Facilitating a Stable Soil Bacterial Community in Compacted Soil. Soil. Tillage Res. 2023, 230, 105686. [Google Scholar] [CrossRef]
- Wang, R.; Shi, W.; Li, Y. Link Between Aeration in the Rhizosphere and P-Acquisition Strategies: Constructing Efficient Vegetable Root Morphology. Front. Environ. Sci. 2022, 10, 906893. [Google Scholar] [CrossRef]
- Carrasco, G.; Gajardo, J.M.; Álvaro, J.E.; Urrestarazu, M. Rocket Production (Eruca sativa Mill.) in a Floating System Using Peracetic Acid as Oxygen Source Compared with Substrate Culture. J. Plant Nutr. 2011, 34, 1397–1401. [Google Scholar] [CrossRef]
- Du, Y.D.; Niu, W.Q.; Gu, X.B.; Zhang, Q.; Cui, B.J.; Zhao, Y. Crop Yield and Water Use Efficiency under Aerated Irrigation: A Meta-Analysis. Agric. Water Manag. 2018, 210, 158–164. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Niu, W.; Wang, J.; Zhang, M. Effect of Post-Infiltration Soil Aeration at Different Growth Stages on Growth and Fruit Quality of Drip-Irrigated Potted Tomato Plants (Solanum lycopersicum). PLoS ONE 2015, 10, e0143322. [Google Scholar] [CrossRef] [PubMed]
- Bonachela, S.; Quesada, J.; Acuña, R.A.; Magán, J.J.; Marfà, O. Oxyfertigation of a Greenhouse Tomato Crop Grown on Rockwool Slabs and Irrigated with Treated Wastewater: Oxygen Content Dynamics and Crop Response. Agric. Water Manag. 2010, 97, 433–438. [Google Scholar] [CrossRef]
- Baram, S.; Weinstein, M.; Evans, J.F.; Berezkin, A.; Sade, Y.; Ben-Hur, M.; Bernstein, N.; Mamane, H. Drip Irrigation with Nanobubble Oxygenated Treated Wastewater Improves Soil Aeration. Sci. Hortic. 2022, 291, 110550. [Google Scholar] [CrossRef]
- Ben-Noah, I.; Friedman, S.P. Aeration of Clayey Soils by Injecting Air through Subsurface Drippers: Lysimetric and Field Experiments. Agric. Water Manag. 2016, 176, 222–233. [Google Scholar] [CrossRef]
- Friedman, S.P.; Naftaliev, B. A Survey of the Aeration Status of Drip-Irrigated Orchards. Agric. Water Manag. 2012, 115, 132–147. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated Subsurface Irrigation Water Gives Growth and Yield Benefits to Zucchini, Vegetable Soybean and Cotton in Heavy Clay Soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Midmore, D.J.; Pendergast, L. Yield, Water-Use Efficiencies and Root Distribution of Soybean, Chickpea and Pumpkin under Different Subsurface Drip Irrigation Depths and Oxygation Treatments in Vertisols. Irrig. Sci. 2008, 26, 439–450. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.; Song, L.; Chen, H. Aerated Irrigation Promotes Soil Respiration and Microorganism Abundance around Tomato Rhizosphere. Soil. Sci. Soc. Am. J. 2019, 83, 1343–1355. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and Applications of Microbubble and Nanobubble Technology for Water Treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and Applications of Nanobubbles: A Review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Kusalik, P.G.; English, N.J. Massive Generation of Metastable Bulk Nanobubbles in Water by External Electric Fields. Sci. Adv. 2020, 6, eaaz0094. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, S.; Cheng, L.; Ma, H.; Gao, X.; Brennan, C.S.; Yan, J.-K. Micro-Nano-Bubble Technology and Its Applications in Food Industry: A Critical Review. Food Rev. Int. 2022, 39, 4213–4235. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk Nanobubbles in the Mineral and Environmental Areas: Updating Research and Applications. Adv. Colloid. Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, K.R.; Ling, L.; Wongkiew, S.; Nhan, H.T.; Surendra, K.C.; Shitanaka, T.; Lu, H.; Khanal, S.K. Nanobubble Technology Applications in Environmental and Agricultural Systems: Opportunities and Challenges. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1378–1403. [Google Scholar] [CrossRef]
- Salinas, J.; Meca, D.; del Moral, F. Short-Term Effects of Changing Soil Management Practices on Soil Quality Indicators and Crop Yields in Greenhouses. Agronomy 2020, 10, 582. [Google Scholar] [CrossRef]
- Fernández, M.D.; Baeza, E.; Céspedes, A.; Pérez-Parra, J.; Gázquez, J.C. Validation Of On-Farm Crop Water Requirements (Prho) Model for Horticultural Crops in an Unheated Plastic Greenhouse. Acta Hortic. 2009, 807, 295–300. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Fernández, M.D.; del Moral, F.; Thompson, R.B.; Gallardo, M. Responses of Soil Properties, Crop Yield and Root Growth to Improved Irrigation and N Fertilization, Soil Tillage and Compost Addition in a Pepper Crop. Sci. Hortic. 2017, 225, 422–430. [Google Scholar] [CrossRef]
- Eklund, F.; Alheshibri, M.; Swenson, J. Differentiating Bulk Nanobubbles from Nanodroplets and Nanoparticles. Curr. Opin. Colloid. Interface Sci. 2021, 53, 101427. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Luna Magdaleno, A.; Graf, J.C.; Apul, O.G.; Garcia-Segura, S. Elucidating CO2 Nanobubble Interfacial Reactivity and Impacts on Water Chemistry. J. Colloid. Interface Sci. 2021, 607, 720–728. [Google Scholar] [CrossRef]
- Mingorance, M.D.D.; Barahona, E.; Fernández-Gálvez, J. Guidelines for Improving Organic Carbon Recovery by the Wet Oxidation Method. Chemosphere 2007, 68, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, R.L. Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 9780891188667. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Available Phosphorus; FAO, Ed.; FAO: Rome, Italy, 2021. [Google Scholar]
- Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Farneselli, M.; Padilla, F.M. Assessing Crop N Status of Fertigated Vegetable Crops Using Plant and Soil Monitoring Techniques. Ann. Appl. Biol. 2015, 167, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, G.; Hochmuth, R. Plant Petiole Sap-Testing for Vegetable Crops. EDIS 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Vignoni, L.A.; Césari, R.M.; Forte, M.; Mirábile, M.L. Determinación de Indice de Color En Ajo Picado. Inf. Tecnológica 2006, 17, 63–67. [Google Scholar] [CrossRef]
- Benito-Bautista, P.; Arellanes-Juárez, N.; Pérez-Flores, M.E. Color y Estado de Madurez Del Fruto de Tomate de Cáscara. Agron. Mesoam. 2015, 27, 115. [Google Scholar] [CrossRef]
- Ashebir, D.; Jezik, K.; Weingartemann, H.; Gretzmacher, R. Change in Color and Other Fruit Quality Characteristics of Tomato Cultivars after Hot-Air Drying at Low Final-Moisture Content. Int. J. Food Sci. Nutr. 2009, 60, 308–315. [Google Scholar] [CrossRef]
- Barreiro, J.A.; Milano, M.; Sandoval, A.J. Kinetics of Colour Change of Double Concentrated Tomato Paste during Thermal Treatment. J. Food Eng. 1997, 33, 359–371. [Google Scholar] [CrossRef]
- Soare, R.; Dinu, M.; Apahidean, A.-I.; Soare, M. The Evolution of Some Nutritional Parameters of the Tomato Fruit during the Harvesting Stages. Hortic. Sci. 2019, 46, 132–137. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of Lycopene, Antioxidant Activity, Total Soluble Solids and Weight Loss of Tomato during Postharvest Storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Donald, C.M.; Hamblin, J. The Biological Yield and Harvest Index of Cereals as Agronomic and Plant Breeding Criteria. Adv. Agron. 1976, 28, 361–405. [Google Scholar]
- Sukor, A.; Qian, Y.; Davis, J.G. Organic Nitrogen Fertilizer Selection Influences Water Use Efficiency in Drip-Irrigated Sweet Corn. Agriculture 2023, 13, 923. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Baram, S.; Evans, J.F.; Berezkin, A.; Ben-Hur, M. Irrigation with Treated Wastewater Containing Nanobubbles to Aerate Soils and Reduce Nitrous Oxide Emissions. J. Clean. Prod. 2021, 280, 124509. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wang, T.; Pan, J.; Zhou, B.; Muhammad, T.; Zhou, C.; Li, Y. Micro-Nano Bubble Water Oxygation: Synergistically Improving Irrigation Water Use Efficiency, Crop Yield and Quality. J. Clean. Prod. 2019, 222, 835–843. [Google Scholar] [CrossRef]
- Wu, Y.; Lyu, T.; Yue, B.; Tonoli, E.; Verderio, E.A.M.; Ma, Y.; Pan, G. Enhancement of Tomato Plant Growth and Productivity in Organic Farming by Agri-Nanotechnology Using Nanobubble Oxygation. J. Agric. Food Chem. 2019, 67, 10823–10831. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bastida, F.; Liu, Y.; Zhou, Y.; He, J.; Song, P.; Kuang, N.; Li, Y. Nanobubble Oxygenated Increases Crop Production via Soil Structure Improvement: The Perspective of Microbially Mediated Effects. Agric. Water Manag. 2023, 282, 108263. [Google Scholar] [CrossRef]
- Zhu, Y.; Dyck, M.; Cai, H.J.; Song, L.B.; Chen, H. The Effects of Aerated Irrigation on Soil Respiration, Oxygen, and Porosity. J. Integr. Agric. 2019, 18, 2854–2868. [Google Scholar] [CrossRef]
- Bonachela, S.; Fernández, M.D.; Cabrera, F.J.; Granados, M.R. Soil Spatio-Temporal Distribution of Water, Salts and Nutrients in Greenhouse, Drip-Irrigated Tomato Crops Using Lysimetry and Dielectric Methods. Agric. Water Manag. 2018, 203, 151–161. [Google Scholar] [CrossRef]
- Bonachela, S.; Acuña, R.A.; Casas, J. Environmental Factors and Management Practices Controlling Oxygen Dynamics in Agricultural Irrigation Ponds in a Semiarid Mediterranean Region: Implications for Pond Agricultural Functions. Water Res. 2007, 41, 1225–1234. [Google Scholar] [CrossRef]
- Grasso, R.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Padilla, F.M. Tillage Effects on Soil Properties, Crop Responses and Root Density of Sweet Pepper (Capsicum Annuum). Span. J. Agric. Res. 2021, 19, e0902. [Google Scholar] [CrossRef]
- Niu, W.; Jia, Z.; Zhang, X.; Shao, H. Effects of Soil Rhizosphere Aeration on the Root Growth and Water Absorption of Tomato. Clean (Weinh) 2012, 40, 1364–1371. [Google Scholar] [CrossRef]
- Rasmussen, I.S.; Dresbøll, D.B.; Thorup-Kristensen, K. Winter Wheat Cultivars and Nitrogen (N) Fertilization—Effects on Root Growth, N Uptake Efficiency and N Use Efficiency. Eur. J. Agron. 2015, 68, 38–49. [Google Scholar] [CrossRef]
- Birlanga, V.; Acosta-Motos, J.R.; Pérez-Pérez, J.M. Mitigation of Calcium-Related Disorders in Soilless Production Systems. Agronomy 2022, 12, 644. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Wu, K.; Wu, S.; Wu, K.; Li, Y.; Niu, W. Micro/Nanobubble-Aerated Drip Irrigation Affects Saline Soil Microenvironments and Tomato Growth by Altering Bacterial Communities. Soil. Tillage Res. 2024, 239, 106034. [Google Scholar] [CrossRef]
- Zhou, Y.; Bastida, F.; Liu, Y.; He, J.; Chen, W.; Wang, X.; Xiao, Y.; Song, P.; Li, Y. Impacts and Mechanisms of Nanobubbles Level in Drip Irrigation System on Soil Fertility, Water Use Efficiency and Crop Production: The Perspective of Soil Microbial Community. J. Clean. Prod. 2021, 333, 130050. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.L.; Fernie, A.R. An Update on Source-to-Sink Carbon Partitioning in Tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of Low Nitrogen Supply on Tomato (Solanum lycopersicum) Fruit Yield and Quality with Special Emphasis on Sugars, Acids, Ascorbate, Carotenoids, and Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.P.; Gangaprasad, S.; Adivappar, N. Genetic Variability, Trait Inter-Relationships, Third and Fourth Degree Statistics Based Genetics for Fruit Quality and Yield Traits Governing Shelf Life in Tomato (Solanum lycopersicum L.). Plant Genet. Resour. 2022, 20, 366–375. [Google Scholar] [CrossRef]
- Cvikić, D.; Zdravković, J.; Pavlović, N.; Adžić, S.; Dordević, M. Postharvest Shelf Life of Tomato (Lycopersicon esculentum Mill.) Mutanats (nor and Rin) and Their Hybrids. Genetika 2012, 44, 449–456. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of Physico-Chemical and Organoleptic Fruit Parameters Relevant for Tomato Quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.J.; Song, L.B.; Shang, Z.H.; Chen, H. Comprehensive Evaluation of Different Oxygation Treatments Based on Fruit Yield and Quality of Greenhouse Tomato. Sci. Agric. Sin. 2020, 53, 2241–2252. [Google Scholar]
- Zhou, Y.; Zhou, B.; Xu, F.; Muhammad, T.; Li, Y. Appropriate Dissolved Oxygen Concentration and Application Stage of Micro-Nano Bubble Water Oxygation in Greenhouse Crop Plantation. Agric. Water Manag. 2019, 223, 105713. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of Environmental Factors and Agricultural Techniques on Antioxidant Content of Tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Z.; Ding, Z.; Yao, K.; Yang, Y.; Hou, X.; Li, C.; Zhang, H.; Li, Y.; Wang, C.; et al. Hydrogen-Rich Water Irrigation Promotes Fruit Ripening and Nutritional Composition in Tomato. Postharvest Biol. Technol. 2024, 213, 112920. [Google Scholar] [CrossRef]







| Control | MNBA | MNBO | |
|---|---|---|---|
| TVW | 5194 | 4825 | 5065 |
| N-NO3 | 573.4 | 565.1 | 583.3 |
| N-NH4 | 25.5 | 24.1 | 24.8 |
| K+ | 640.6 | 681.3 | 708.1 |
| Ca++ | 926.1 | 865.4 | 901.3 |
| Mg++ | 388.9 | 360.7 | 378.8 |
| SO4= | 395.9 | 423.7 | 440.3 |
| HCO3− | 126.5 | 126.6 | 131.0 |
| Na+ | 980.7 | 909.6 | 955.4 |
| Cl− | 2822.7 | 2618.0 | 2749.8 |
| Fe | 7.0 | 6.5 | 6.8 |
| Mn | 4.0 | 3.7 | 3.9 |
| Zn | 1.6 | 1.4 | 1.5 |
| B | 1.3 | 1.2 | 1.3 |
| Cu | 0.3 | 0.3 | 0.3 |
| MNBO | MNBA | |
|---|---|---|
| Mean (nm) | 161.7 ± 4.5 | 174.1 ± 32.9 |
| Mode (nm) | 96.1 ± 2.8 | 125.2 ± 32.2 |
| D10 (nm) | 89.7 ± 2.9 | 110.6 ± 30.1 |
| D50 (nm) | 134.6 ± 3.8 | 144.3 ± 34.1 |
| D90 (nm) | 290.9 ± 8.4 | 268.8 ± 27.8 |
| Concentration (particles mL−1) | 2.5 × 108 ± 1.7 × 107 | 1.93 × 108 ± 4.09 × 107 |
| Control | MNBA | MNBO | |
|---|---|---|---|
| Tank or pond (before irrigation) | 5.6 ± 0.7 | 7.6 ± 0.3 | 29.78 ± 1.17 |
| Irrigation solution | 4.51 ± 0.6 | 7.0 ± 0.2 | 24.2 ± 1.7 |
| Sampling Time T1 | Sampling Time T2 | |||
|---|---|---|---|---|
| Control | MNBA | MNBO | ||
| TOC (g kg−1) | 10.49 ± 2.77 a | 6.7 ± 1.3 b | 6.3 ± 1.2 b | 4.4 ± 1.8 b |
| pH | 8.53 ± 0.31 | 8.57 ± 0.21 | 8.53 ± 0.15 | 8.53 ± 0.12 |
| EC (dS m−1) | 0.77 ± 0. 4 a | 0.53 ± 0.09 b | 0.60 ± 0.02 b | 0.52 ± 0.0 b |
| P (g kg−1) | 0.21 ± 0.11 a | 0.16 ± 0.01 b | 0.14 ± 0.04 b | 0.15 ± 0.03 b |
| K (g kg−1) | 2.40 ± 0.69 | 2.33 ± 0.58 | 2.83 ± 0.58 | 2.17 ± 0.29 |
| Ntot (g kg−1) | 1.19 ± 0.29 | 1.08 ± 0.15 | 0.93 ± 0.11 | 1.00 ± 0.26 |
| Nmin (mg kg−1) | 25.62 ± 17.7 a | 14.62 ± 5.79 b | 15.45 ± 6.86 b | 13.89 ± 4.46 b |
| N-NO3 (mg kg−1) | 17.48 ± 15.84 a | 8.37 ± 7.67 b | 8.51 ± 5.04 b | 7.64 ± 2.21 b |
| N-NH4 (mg kg−1) | 8.14 ± 5.69 a | 6.25 ± 3.67 b | 6.94 ± 2.18 b | 6.25 ± 3.7 b |
| NO3s− (mg L−1) | 351 ± 134 a | 317 ± 61 b | 330 ± 157 b | 277 ± 55 b |
| Ks+ (mg L−1) | 62.5 ± 41.0 a | 27.3 ± 1.5 b | 24.0 ± 5.1 b | 26.3 ± 5.7 b |
| Sand (% w/w) | 45.3 ± 2.8 | - | - | - |
| Silt (% w/w) | 34.3 ± 3.0 | - | - | - |
| Clay (% w/w) | 20.4 ± 1.0 | - | - | - |
| Control | MNBA | MNBO | |
|---|---|---|---|
| Total | 113.02 ± 2.43 | 118.79 ± 6.97 | 117.79 ± 3.8 |
| Marketable | 97.2 ± 3.82 | 103.65 ± 8.73 | 103.57 ± 5 |
| Non-marketable | 15.82 ± 2.01 | 15.14 ± 1.88 | 14.21 ± 1.44 |
| Class I | 67.14 ± 4.1 | 68.94 ± 5.63 | 70.91 ± 3.9 |
| Class II | 28.08 ± 3.14 | 32.18 ± 3.62 | 31.99 ± 3.2 |
| Total Dry weight | 10.57 ± 0.87 | 10.43 ± 0.50 | 10.18 ± 0.68 |
| Ratio Fresh/Dry weight | 10.69 | 11.39 | 11.57 |
| Time 1: 18/2/2022 | Time 2: 5/4/2022 | |||||
|---|---|---|---|---|---|---|
| Control | MNBA | MNBO | Control | MNBA | MNBO | |
| Fresh weight | 116.36 ± 3.2 b | 107.5 ± 2.47 b | 145.42 ± 7.91 a | 67.22 ±3.75 b | 78.84 ± 4.50 a | 72.00 ± 7.10 ab |
| pH | 3.87 ± 0.15 | 3.81 ± 0.12 | 3.88 ± 0.12 | 4.36 ± 0.04 | 4.44 ± 0.1 | 4.36 ± 0.05 |
| Titratable acidity | 0.47 ± 0.04 | 0.47 ± 0.02 | 0.46 ± 0.02 | 0.54 ±0.03 a | 0.49 ± 0.02 b | 0.53 ± 0.03 ab |
| Soluble solids | 5.43 ± 0.15 | 5.57 ± 0.20 | 5.20 ± 0.06 | 6.68 ± 0.17 a | 5.48 ± 0.39 b | 6.33 ± 0.44 ab |
| Maturity index | 11.8 ± 1.12 | 11.86 ± 0.79 | 11.27 ± 0.41 | 12.35 ± 0.34 | 11.21 ± 0.63 | 11.8 ± 0.34 |
| Axial diameter | 60.35 ± 1.27 | 60.04 ± 0.95 | 61.71 ± 1.52 | 52.44 ±1.06 b | 57.36 ± 1.89 a | 53.19 ± 2.31 b |
| Equatorial diameter | 58.48 ± 0.64 b | 56.45 ± 0.84 b | 63.8 ± 1.18 a | 47.95 ±1.18 b | 50.24 ± 1.31 a | 47.97 ± 2.07 b |
| Hardness | 67.00 ± 1.5 | 70.40 ± 1.5 | 72.40 ± 3.5 | 85.80 ± 3.9 | 88.00 ± 1.7 | 89.10 ± 1.9 |
| Firmness | 3.30 ± 0.08 | 3.55 ± 0.1 | 3.71 ± 0.27 | 4.15 ± 0.09 | 4.08 ± 0.11 | 4.15 ± 0.11 |
| Wall thickness | 6.13 ± 0.39 b | 6.67 ± 0.43 a | 6.48 ± 0.47 ab | |||
| L | 37.55 ± 0.5 | 37.28 ± 0.56 | 37.05 ± 0.57 | 37.72 ± 1.6 | 38.42 ± 1.25 | 36.09 ± 2.37 |
| a | 15.64 ± 1.09 | 15.95 ± 1.36 | 17.48 ± 1.04 | 21.55 ± 1.16 | 22.72 ± 0.22 | 20.5 ± 1.25 |
| b | 15.28 ± 0.64 | 15.86 ± 0.35 | 15.69 ± 0.25 | 16.65 ± 0.56 | 17.03 ± 0.36 | 15.48 ± 1.23 |
| Colour index | 27.88 ± 2.72 | 27.39 ± 2.72 | 30.47 ± 2.21 | 34.4 ± 1.43 | 34.9 ± 1.49 | 37.73 ± 4.13 |
| ΔE MNBx-Control | 1.47 ± 0.28 | 3.34 ± 0.82 | 4.06 ± 2.2 | 2.28 ± 1.95 | ||
| Harvest Index | ANUE | AKUE | fWUE (kg m−3) | |
|---|---|---|---|---|
| Control | 73.69 | 123.44 | 111.37 | 21.76 |
| MNBA | 74.25 | 131.11 | 112.54 | 24.62 |
| MNBO | 71.71 | 127.36 | 111.83 | 23.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Moral Torres, F.; Hernández Maqueda, R.; Meca Abad, D.E. Enhancing Root Distribution, Nitrogen, and Water Use Efficiency in Greenhouse Tomato Crops Using Nanobubbles. Horticulturae 2024, 10, 463. https://doi.org/10.3390/horticulturae10050463
del Moral Torres F, Hernández Maqueda R, Meca Abad DE. Enhancing Root Distribution, Nitrogen, and Water Use Efficiency in Greenhouse Tomato Crops Using Nanobubbles. Horticulturae. 2024; 10(5):463. https://doi.org/10.3390/horticulturae10050463
Chicago/Turabian Styledel Moral Torres, Fernando, Rafael Hernández Maqueda, and David Erik Meca Abad. 2024. "Enhancing Root Distribution, Nitrogen, and Water Use Efficiency in Greenhouse Tomato Crops Using Nanobubbles" Horticulturae 10, no. 5: 463. https://doi.org/10.3390/horticulturae10050463