Enhancing Transparency in Non-Cubic Calcium Phosphate Ceramics: Effect of Starting Powder, LiF Doping, and Spark Plasma Sintering Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powders Used for Sintering via SPS Method
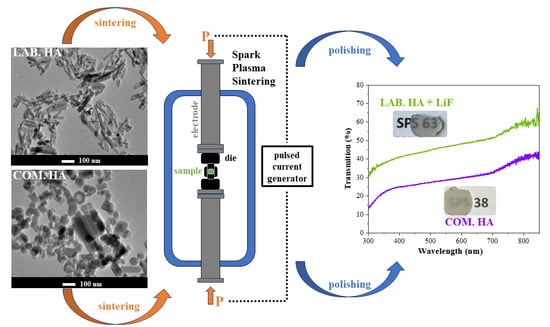
2.2. Sintering by SPS
2.3. Analysis and Characterization Techniques
2.3.1. Microscopic Analysis of Starting Powders by TEM
2.3.2. Microscopic Analysis of Sintered Ceramics by SEM
2.3.3. Disc Polishing
2.3.4. Density Measurements
2.3.5. Transmission Measurements of Sintered Ceramics
3. Results
3.1. Raw Nanopowder Characterizations
3.1.1. Phase Analysis
3.1.2. Morphology and Particle Size of Nanopowders by TEM Analysis
3.2. Characterizations of Micro-Ceramics Fabricated by SPS
3.2.1. Influence of LiF Doping
3.2.2. Influence of Temperature of SPS
3.2.3. Influence of the Starting Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikesue, A.; Aung, L.A. (Eds.) Processing of Ceramics: Breakthroughs in Optical Materials; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Goldstein, A.; Krell, A. Transparent ceramics at 50: Progress made and further prospects. J. Am. Ceram. Soc. 2016, 99, 3173–3197. [Google Scholar] [CrossRef]
- Goldstein, A.; Andreas Krell, A.; Burshtein, Z. Transparent Ceramics: Materials, Engineering, and Applications; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-42949-4. [Google Scholar]
- Krell, A.; Hutzler, T.; Klimke, J. Transmission physics and consequences for materials selection, manufacturing, and applications. J. Eur. Ceram. Soc. 2009, 29, 207–222. [Google Scholar] [CrossRef]
- Hostaša, J.; Picelli, F.; Hříbalová, S.; Nečina, V. Sintering aids, their role and behaviour in the production of transparent ceramics. Open Ceram. 2021, 7, 100137. [Google Scholar] [CrossRef]
- Frage, N.; Cohen, S.; Meir, S.; Kalabukhov, S.; Dariel, M.P. Spark plasma sintering (SPS) of transparent magnesium-aluminate spinel. J. Mater. Sci. 2007, 42, 3273–3275. [Google Scholar] [CrossRef]
- Nečina, V.; Pabst, W. Comparison of the effect of different alkali halides on the preparation of transparent MgAl2O4 spinel ceramics via spark plasma sintering (SPS). J. Eur. Ceram. Soc. 2020, 40, 6043–6052. [Google Scholar] [CrossRef]
- Ikesue, A.; Kinoshita, T.; Kamata, K.; Yoshida, K. Fabrication and optical properties of high-performance polycrystalline Nd: YAG ceramics for solid-state lasers. J. Am. Ceram. Soc. 1995, 78, 1033–1040. [Google Scholar] [CrossRef]
- Yagi, H.; Yanagitani, Y.; Takaichi, K.; Ueda, K.; Kaminskii, A. Characterizations and laser performances of highly transparent Nd3+:Y3Al5O12 laser ceramics. Opt. Mater. 2007, 29, 1258–1262. [Google Scholar] [CrossRef]
- Talimian, A.; Pouchly, V.; El-Maghraby, H.F.; Maca, K.; Galusek, D. Transparent magnesium aluminate spinel: Effect of critical temperature in two-stage spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 2417–2425. [Google Scholar] [CrossRef]
- Grasso, S.; Yoshida, H.; Porwal, H.; Sakka, Y.; Reece, M. Highly transparent α-alumina obtained by low cost high pressure SPS. Ceram. Int. 2013, 39, 3243–3248. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H. Spark plasma sintering of transparent alumina. Scr. Mater. 2007, 57, 607–610. [Google Scholar] [CrossRef]
- Sarthou, J.; Aballea, P.; Patriarche, G.; Serier-Brault, H.; Suganuma, A.; Gredin, P.; Mortier, M.; Riman, R. Wet-route synthesis and characterization of Yb:CaF2 optical ceramics. J. Am. Ceram. Soc. 2016, 99, 1992–2000. [Google Scholar] [CrossRef]
- Ivanov, M.; Kalinina, E.; Kopylov, Y.; Kravchenko, V.; Krutikova, I.; Kynast, U.; Li, J.; Leznina, M.; Medvedev, A. Highly transparent Yb-doped (LaxY1−x)2O3 ceramics prepared through colloidal methods of nanoparticles compaction. J. Eur. Ceram. Soc. 2016, 36, 4251–4259. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, L.A.; Lupei, V. Ceramic Lasers; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Sobota, P.; Guzik, M.; Garnier, V.; Fantozzi, G.; Sobota, M.; Tomaszewicz, E.; Guyot, Y.; Boulon, G. Fabrication of Y6MoO12 molybdate ceramics: From synthesis of cubic nanopowder to sintering. Ceram. Int. 2020, 46, 4619–4633. [Google Scholar] [CrossRef]
- Wilk (Bieza), M.; Tomaszewicz, E.; Siczek, M.; Guyot, Y.; Boulon, G.; Guzik, M. The first characterization of cubic Nd3+-doped mixed La2MoWO9 in micro-crystalline powders and translucent micro-ceramics. J. Mater. Chem. C 2022, 10, 10083–10098. [Google Scholar] [CrossRef]
- Bieza, M.; Guzik, M.; Tomaszewicz, E.; Guyot, Y.; Lebbou, K.; Zych, E.; Boulon, G. Toward optical ceramics based on cubic Yb3+ rare earth ion-doped mixed molybdato- tungstates: Part I—Structural characterization. J. Phys. Chem. C 2017, 121, 13290–13302. [Google Scholar] [CrossRef]
- Bieza, M.; Guzik, M.; Tomaszewicz, E.; Guyot, Y.; Lebbou, K.; Boulon, G. Toward optical ceramics based on cubic Yb3+ rare earth ion-doped mixed molybdate-tungstates: Part II—Spectroscopic characterization. J. Phys. Chem. C 2017, 121, 13303–13313. [Google Scholar] [CrossRef]
- Prokop, K.A.; Siczek, M.; Tomaszewicz, E.; Rola, K.; Guyot, Y.; Boulon, G.; Guzik, M. Structural ordering studies of Nd3+ ion in cubic M3Y(PO4)3 (M = Sr2+ or Ba2+) perovskites. First translucent ceramics from micro-crystalline cubic powders. Ceram. Int. 2024, 50, 8042–8056. [Google Scholar] [CrossRef]
- Kawagoe, D.; Ioku, K.; Fujimori, H.; Goto, S. Transparent β-tricalcium phosphate ceramics prepared by spark plasma sintering. J. Ceram. Soc. Jpn. 2004, 112, 462–463. [Google Scholar] [CrossRef]
- Kim, B.N.; Horiuchi, N.; Dash, A.; Kim, Y.W.; Morita, K.; Yoshida, H.; Ji-Guang, L.; Sakka, Y. Spark plasma sintering of highly transparent hydroxyapatite ceramics. J. Jpn. Soc. Powder Powder Metall. 2017, 64, 547–551. [Google Scholar] [CrossRef]
- Furuse, H.; Kato, D.; Morita, K.; Suzuki, T.S.; Kim, B.N. Characterization of Transparent Fluorapatite Ceramics Fabricated by Spark Plasma Sintering. Materials 2022, 15, 8157. [Google Scholar] [CrossRef]
- Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Optical and X-ray-induced luminescence properties of calcium phosphate of transparent ceramic form. Sens. Mater. 2020, 32, 1411–1417. [Google Scholar] [CrossRef]
- Ioku, K.; Kamitakahara, M. Hydroxyapatite ceramics for medical application prepared by hydrothermal method. Phosphorus Res. Bull. 2009, 23, 25–30. [Google Scholar] [CrossRef]
- Kotobuki, N.; Kawagoe, D.; Nomura, D.; Katou, Y.; Muraki, K.; Jujimori, H.; Goto, S.; Ioku, K.; Ohgushi, H. Observation and quantitative analysis of rat bone marrow stromal cells cultured in vitro on newly formed transparent β-tricalcium phosphate. J. Mater. Sci. Mater Med. 2006, 17, 33–41. [Google Scholar] [CrossRef]
- Akiyama, J.; Sato, Y.; Taira, T. Laser ceramics with rare-earth-doped anisotropic materials. Opt. Lett. 2010, 35, 3598–3600. [Google Scholar] [CrossRef]
- Akiyama, J.; Sato, Y.; Taira, T. Laser demonstration of diode-pumped Nd3+-doped fluorapatite anisotropic ceramics. Appl. Phys. Express. 2011, 4, 022703-1–022703-3. [Google Scholar] [CrossRef]
- Furuse, H.; Horiuchi, N.; Kim, B.N. Transparent non-cubic laser ceramics with fine microstructure. Sci. Rep. 2019, 9, 2–3. [Google Scholar] [CrossRef]
- Furuse, H.; Okabe, T.; Shirato, H.; Kato, D.; Horiuchi, N.; Morita, K.; Kim, B.N. High-optical-quality non-cubic Yb3+-doped Ca10(PO4)6F2 (Yb:FAP) laser ceramics. Opt. Mater. Express 2021, 11, 1756. [Google Scholar] [CrossRef]
- Eriksson, M.; Liu, Y.; Hu, J.; Gao, L.; Nygren, M.; Shen, Z. Transparent hydroxyapatite ceramics with nanograin structure prepared by high-pressure spark plasma sintering at the minimized sintering temperature. J. Eur. Ceram. Soc. 2011, 31, 1533–1540. [Google Scholar] [CrossRef]
- Rubat du Merac, M.; Kleebe, H.J.; Müller, M.M.; Reimanis, I.E. Fifty years of research and development coming to fruition; Unraveling the complex interactions during processing of transparent magnesium aluminate (MgAl2O4) spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Frage, N.; Kalabukhov, S.; Sverdlov, N.; Ezersky, V.; Dariel, M.P. Densification of transparent yttrium aluminum garnet (YAG) by SPS processing. J. Eur. Ceram. Soc. 2010, 30, 3331–3337. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, X.; Wang, J.; Liu, P.; Li, D.; Wang, X.; Zhang, J.; Xu, J.; Tang, D. Fabrication and spectral properties of Dy:Y2O3 transparent ceramics. J. Eur. Ceram. Soc. 2018, 38, 1981–1985. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, X.; Wang, J.; Liu, P.; Li, D.; Wang, X.; An, L.; Zhang, J.; Xu, J.; Tang, D. Spark plasma sintering of Sm3+ doped Y2O3 transparent ceramics for visible light lasers. Ceram. Int. 2017, 43, 12057–12060. [Google Scholar] [CrossRef]
- Alombert-Goget, G.; Guyot, Y.; Guzik, M.; Boulon, G.; Ito, A.; Goto, T.; Yoshikawa, A.; Kikuchi, M. Nd3+-doped Lu2O3 transparent sesquioxide ceramics elaborated by the Spark Plasma Sintering (SPS) method. Part 1: Structural, thermal conductivity and spectroscopic characterization. Opt. Mater. 2015, 41, 3–11. [Google Scholar] [CrossRef]
- Jiang, N.; Xie, R.-J.; Liu, Q.; Li, J. Fabrication of sub-micrometer MgO transparent ceramics by spark plasma sintering. J. Eur. Ceram. Soc. 2017, 37, 4947–4953. [Google Scholar] [CrossRef]
- Jokić, B.; Mitrić, M.; Radmilović, V.; Drmanić, S.; Petrović, R.; Janaćković, D. Synthesis and characterization of monetite and hydroxyapatite whiskers obtained by a hydrothermal method. Ceram. Int. 2011, 37, 167–173. [Google Scholar] [CrossRef]
- Syukkalova, E.A.; Sadetskaya, A.V.; Demidova, N.D.; Bobrysheva, N.P.; Osmolowsky, M.G.; Voznesenskiy, M.A.; Osmolovskaya, O.M. The effect of reaction medium and hydrothermal synthesis conditions on morphological parameters and thermal behavior of calcium phosphate nanoparticles. Ceram. Int. 2021, 47, 2809–2821. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Tsuru, K.; Garskaite, E.; Stankeviciute, Z.; Beganskiene, A.; Ishikawa, K.; Kareiva, A. A novel wet polymeric precipitation synthesis method for monophasic β-TCP. Adv. Powder Technol. 2017, 28, 2325–2331. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Wang, X.; Chen, X. Synthesis and sintering of nanocrystalline hydroxyapatite powders by citric acid sol-gel combustion method. Mater. Res. Bull. 2004, 39, 25–32. [Google Scholar] [CrossRef]
- He, W.; Xie, Y.; Xing, Q.; Ni, P.; Han, Y.; Dai, H. Sol-gel synthesis of biocompatible Eu3+/Gd3+ co-doped calcium phosphate nanocrystals for cell bioimaging. J. Lumin. 2017, 192, 902–909. [Google Scholar] [CrossRef]
- Anee, T.K.; Ashok, M.; Palanichamy, M.; Kalkura, S.N. A novel technique to synthesize hydroxyapatite at low temperature. Mater. Chem. Phys. 2003, 80, 725–730. [Google Scholar] [CrossRef]
- Han, J.K.; Song, H.Y.; Saito, F.; Lee, B.T. Synthesis of high purity nano-sized hydroxyapatite powder by microwave-hydrothermal method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Kalita, S.J.; Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C 2010, 30, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kumta, P.N. Mechano-chemical synthesis and characterization of nanostructured β-TCP powder. Mater. Sci. Eng. C 2007, 27, 377–381. [Google Scholar] [CrossRef]
- Bricha, M.; Belmamouni, Y.; Essassi, E.M.; Ferreira, J.M.F.; El Mabrouk, K. Surfactant-assisted hydrothermal synthesis of hydroxyapatite nanopowders. J. Nanosci. Nanotechnol. 2012, 12, 8042–8049. [Google Scholar] [CrossRef] [PubMed]
- Bilton, M.; Milne, S.J.; Brown, A.P. Comparison of Hydrothermal and Sol-Gel Synthesis of Nano-Particulate Hydroxyapatite by Characterisation at the Bulk and Particle Level. Open J. Inorg. Non Met. Mater. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or Monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]









| LiF wt. % | Phase 1—Main Ca5(PO4)3(OH) Hexagonal P63/m (No 176) HA | Phase 2 Ca3(PO4)2 Trigonal R3c (No 161) β-TCP | Grain Size (µm) | Transmission in % at 650 nm | Transmission in % at 880 nm | Relative Density % |
|---|---|---|---|---|---|---|
| 0 | 71.4% | 28.6% | 0.41–2.1 | 23.4 | 33.8 | 99.0 |
| 0.25 | 77.7% | 22.3% | 0.55–3.2 | 29.7 | 42.8 | 98.8 |
| 0.5 | 81.8% | 18.2% | 2.1–5.4 | 30.5 | 45.6 | 98.8 |
| 1 | 94.5% | 5.5% | 1.8–8.5 | 5.5 | 12.8 | 99.5 |
| Temperature of Sintering x °C/15 min | Phase 1—Main Ca5(PO4)3(OH) HA Hexagonal | Phase 2 Ca3(PO4)2 β-TCP Trigonal | Grain Size (µm) | Transmission in % at 650 nm | Transmission in % at 880 nm | Relative Density % |
|---|---|---|---|---|---|---|
| 950 °C | 77.4% | 22.6% | 0.6–1.5 | 16.8 | 29.2 | 99.4 |
| 1000 °C | 79.3% | 20.7% | 0.7–2.6 | 34.8 | 48.7 | 99.3 |
| 1050 °C | 77.7% | 22.3% | 0.55–3.2 | 29.7 | 42.8 | 98.8 |
| 1100 °C | 71.5% | 28.5% | 0.7–6.4 | 24.5 | 37.2 | 98.9 |
| 1150 °C | 73.9% | 26.1% | 2.1–7.2 | 16.2 | 25.6 | 99.2 |
| Starting Material | LiF wt. % | Temp. of SPS x °C/ 15 min | Phase 1 HA Hexagonal | Phase 2 β-TCP Trigonal | Grain Size (µm) | Transmission in % at 650 nm | Transmission in % at 880 nm | Relative Density % |
|---|---|---|---|---|---|---|---|---|
| COM. HA | 0 | 1050 °C | 70.3% | 29.7% | 0.48–2.1 | 24.1 | 34.4 | 99.0 |
| 0.25 | 77.7% | 22.3% | 0.55–3.2 | 29.7 | 42.8 | 98.8 | ||
| LAB. HA | 0 | 1000 °C | 87.2% | 12.8% | 0.29–0.35 | 35.3 | 45.9 | 100.2 |
| 0.9–1.5 | ||||||||
| 1050 °C | 84.6% | 15.4% | 0.7–2.7 | 33.3 | 45.1 | 99.6 | ||
| 0.25 | 1000 °C | 89.8% | 10.2% | 0.4–1 | 47.3% | 59.1% | 99.8 | |
| 2.2–3.2 | ||||||||
| 1050 °C | 89.4% | 10.6% | 0.5–0.8 | 50% | 59.3% | 99.9 | ||
| 2.4–5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, K.A.; Cottrino, S.; Garnier, V.; Fantozzi, G.; Guyot, Y.; Boulon, G.; Guzik, M. Enhancing Transparency in Non-Cubic Calcium Phosphate Ceramics: Effect of Starting Powder, LiF Doping, and Spark Plasma Sintering Parameters. Ceramics 2024, 7, 607-624. https://doi.org/10.3390/ceramics7020040
Prokop KA, Cottrino S, Garnier V, Fantozzi G, Guyot Y, Boulon G, Guzik M. Enhancing Transparency in Non-Cubic Calcium Phosphate Ceramics: Effect of Starting Powder, LiF Doping, and Spark Plasma Sintering Parameters. Ceramics. 2024; 7(2):607-624. https://doi.org/10.3390/ceramics7020040
Chicago/Turabian StyleProkop, Kacper Albin, Sandrine Cottrino, Vincent Garnier, Gilbert Fantozzi, Yannick Guyot, Georges Boulon, and Małgorzata Guzik. 2024. "Enhancing Transparency in Non-Cubic Calcium Phosphate Ceramics: Effect of Starting Powder, LiF Doping, and Spark Plasma Sintering Parameters" Ceramics 7, no. 2: 607-624. https://doi.org/10.3390/ceramics7020040