Proteases: Importance, Immobilization Protocols, Potential of Activated Carbon as Support, and the Importance of Modifying Supports for Immobilization
1. Introduction
2. Proteases
2.1. Pepsin
2.2. Trypsin
2.3. Papain
2.4. Bromelains
2.5. Ficin
3. Immobilization
3.1. Protease Immobilization
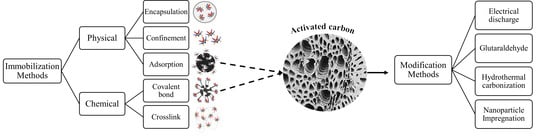
3.1.1. Methods for Protease Immobilization
3.1.2. Supports for Enzyme Immobilization with Potential for Application in the Immobilization of Proteases
3.2. Activated Carbon as a Support for Enzyme Immobilization
3.2.1. Modification of Activated Carbon
3.2.2. Proteases Immobilized on Activated Carbon
4. Immobilized Protease Application
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological Applications of Proteases in Food Technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-activated chitosan matrix for immobilization of a novel cysteine protease, procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef]
- Barbosa, E.E.P.; Pimenta, L.; Brito, A.K.P.; Martim, S.R.; Teixeira, M.F.S.T. Mushroom cultivation edible in lignocellulosic residues from rainforest for protease production. Braz. J. Dev. 2020, 6, 92475–92485. [Google Scholar] [CrossRef]
- Moran, E.T. Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity. Anim. Feed Sci. Technol. 2016, 221, 284–303. [Google Scholar] [CrossRef]
- Souza Júnior, E.C.; Santos, M.P.F.; Sampaio, V.S.; Ferrão, S.P.B.; Fontan, R.C.I.; Bonomo, R.C.F.; Veloso, C.M. Hydrolysis of casein from different sources by immobilized trypsin on biochar: Effect of immobilization method. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1146, 122124. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Enzyme Immobilization: Research and Studies. Int. J. Res. Rev. 2016, 2, 754–757. [Google Scholar]
- Wen-qiong, W.; Lan-wei, Z.; Xue, H.; Yi, L. Cheese whey protein recovery by ultrafiltration through transglutaminase (TG) catalysis whey protein cross-linking. Food Chem. 2017, 215, 31–40. [Google Scholar] [CrossRef]
- Khan, M.; Husain, Q.; Bushra, R. Immobilization of β-galactosidase on surface modified cobalt/multiwalled carbon nanotube nanocomposite improves enzyme stability and resistance to inhibitor. Int. J. Biol. Macromol. 2017, 105, 693–701. [Google Scholar] [CrossRef]
- Khoobi, M.; Motevalizadeh, S.F.; Asadgol, Z.; Forootanfar, H.; Shafiee, A.; Faramarzi, M.A. Synthesis of functionalized polyethylenimine-grafted mesoporous silica spheres and the effect of side arms on lipase immobilization and application. Biochem. Eng. J. 2014, 88, 131–141. [Google Scholar] [CrossRef]
- Santos, M.P.F.; Brito, M.J.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin immobilization on biochar by adsorption and covalent binding, and its application for hydrolysis of bovine casein. J. Chem. Technol. Biotechnol. 2019, 94, 1982–1990. [Google Scholar] [CrossRef]
- Brito, M.J.P.; Veloso, C.M.; Bonomo, R.C.F.; Fontan, R.d.C.I.; Santos, L.S.; Monteiro, K.A. Activated carbons preparation from yellow mombin fruit stones for lipase immobilization. Fuel Process. Technol. 2017, 156, 421–428. [Google Scholar] [CrossRef]
- Gupta, S.; Bhattacharya, A.; Murthy, C.N. Tune to immobilize lipases on polymer membranes: Techniques, factors and prospects. Biocatal. Agric. Biotechnol. 2013, 2, 171–190. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Dos Santos, L.A.; De Almeida, J.M.; Krieger, N.; Mateo, C. Recent trends in biomaterials for immobilization of lipases for application in non-conventional media. Catalysts 2020, 10, 697. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Queissada, D.D.; Silva, J.A. Enzyme immobilization in organic and inorganic supports: Advantages and disadvantages. Holos Environ. 2020, 21, 1–15, Retrieved from Holos Environ. 2020, 20, 271–286. [Google Scholar] [CrossRef]
- Tomke, P.D.; Rathod, V.K. A novel step towards immobilization of biocatalyst using agro waste and its application for ester synthesis. Int. J. Biol. Macromol. 2018, 117, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; De Marco, L.M. Comparative study of the immobilization of pancreatin and papain on activated carbon and alumina, using whey as protein substrate. World Appl. Sci. J. 2007, 2, 175–183. Available online: http://idosi.org/wasj/wasj2(3)/5.pdf (accessed on 25 March 2024).
- Dutta, S.; Bhattacharyya, A.; De, P.; Ray, P.; Basu, S. Removal of mercury from its aqueous solution using charcoal-immobilized papain (CIP). J. Hazard. Mater. 2009, 172, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Karthikeyan, S.; Boopathy, R.; Kennedy, L.J.; Mandal, A.B.; Sekaran, G. Surface functionalized mesoporous activated carbon for the immobilization of acidic lipase and their application to hydrolysis of waste cooked oil: Isotherm and kinetic studies. Process Biochem. 2012, 47, 435–445. [Google Scholar] [CrossRef]
- Cui, C.; Chen, H.; Chen, B.; Tan, T. Genipin Cross-Linked Glucose Oxidase and Catalase Multi-enzyme for Gluconic Acid Synthesis. Appl. Biochem. Biotechnol. 2017, 181, 526–535. [Google Scholar] [CrossRef]
- Furlani, I.L.; Amaral, B.S.; Oliveira, R.V.; Cass, Q.B. Enzyme immobilization: Concepts and effects on proteolysis. Quím. Nova 2020, 43, 463–473. [Google Scholar] [CrossRef]
- Muri, E.M.F. Viral proteases: Important targets of peptidemimetic compounds. Quím. Nova 2014, 37, 308–316. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Soares, R.D.L.; Silva, V.D.M.; Lopes, D.C.F.; Junqueira, R.G.; Figueiredo, A.F.D.S.; Silvestre, M.P.C. Perfil peptídico de hidrolisados enzimáticos de leite em pó desnatado. Braz. J. Pharm. Sci. 2004, 40, 353–362. [Google Scholar] [CrossRef]
- Dornelles, L.P.; de Souza, M.D.; da Silva, P.M.; Procópio, T.F.; Roldan Filho, R.S.; de Albuquerque Lima, T.; de Oliveira, A.P.; Zingali, R.B.; Paiva, P.M.; Pontual, E.V.; et al. Purification and characterization of a protease from the visceral mass of Mytella charruana and its evaluation to obtain antimicrobial peptides. Food Chem. 2018, 245, 1169–1175. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Tang, J.; Pepsin, A. Handbook of Proteolytic Enzymes; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 1, pp. 27–35. [Google Scholar] [CrossRef]
- Szałapata, K.; Osińska-Jaroszuk, M.; Bryjak, J.; Jaszek, M.; Jarosz-Wilkołazka, A. Novel application of porous and cellular materials for covalent immobilization of pepsin. Braz. J. Chem. Eng. 2016, 33, 251–260. [Google Scholar] [CrossRef]
- Miura, Y.; Kageyama, T.; Moriyama, A. Pepsinogens and pepsins from largemouth bass, Micropterus salmoides: Purification and characterization with special reference to high proteolytic activities of bass enzymes. Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2015, 183, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Jin, D.; Meng, M.; Jiang, Y.; Ni, L.; Liu, Z. Immobilization of trypsin onto large-pore mesoporous silica and optimization enzyme activity via response surface methodology. Solid State Sci. 2019, 89, 15–24. [Google Scholar] [CrossRef]
- Amri, E.; Mamboya, F. Papain, a plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Vizovišek, M.; Vidmar, R.; Drag, M.; Fonović, M.; Salvesen, G.S.; Turk, B. Protease Specificity: Towards In Vivo Imaging Applications and Biomarker Discovery. In Trends in Biochemical Sciences; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Kamli, M.R.; Tavano, O.; Fernandez-Lafuente, R. Immobilization of papain: A review. Int. J. Biol. Macromol. 2021, 188, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Singhal, G.; Bhagyawant, S.S.; Srivastava, N. Plant-derived enzymes: A treasure for food biotechnology. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 483–502. [Google Scholar] [CrossRef]
- Guevara, M.G.; Daleo, G.R. Biotechnological Applications of Plant Proteolytic Enzymes; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New trends for a classical enzyme: Papain, a biotechnological success story in the food industry. In Trends in Food Science and Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- de Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Matagne, A.; Bolle, L.; El Mahyaoui, R.; Baeyens-Volant, D.; Azarkan, M. The proteolytic system of pineapple stems revisited: Purification and characterization of multiple catalytically active forms. Phytochemistry 2017, 138, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D. Stem Bromelain. In Handbook of Proteolytic Enzymes; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 1870–1871. [Google Scholar] [CrossRef]
- Ritonja, A.; Rowan, A.D.; Buttle, D.J.; Rawlings, N.D.; Turk, V.; Barrett, A.J. Stem bromelain: Amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989, 247, 419–424. [Google Scholar] [CrossRef]
- Rowan, A.D.; Buttle, D.J.; Barrett, A.J. The cysteine proteinases of the pineapple plant. Biochem. J. 1990, 266, 869–875. [Google Scholar] [PubMed]
- Rowan, A.D. Fruit Bromelain. In Handbook of Proteolytic Enzymes; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 1874–1875. [Google Scholar] [CrossRef]
- Nelson, A.; Peter, A.; Saju, F. A review on chemistry, therapeutic applications, extraction & purification of bromelain. Int. J. Pharmacogn. Chem. 2022, 3, 25–33. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Aznan, T.N.T.; Illias, R.M. Bromelain: From production to commercialisation. J. Sci. Food Agric. 2017, 97, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Baeyens-Volant, D.; Matagne, A.; El Mahyaoui, R.; Wattiez, R.; Azarkan, M. A novel form of ficin from Ficus carica latex: Purification and characterization. Phytochemistry 2015, 117, 154–167. [Google Scholar] [CrossRef]
- Sidek, N.A.A.; Halim, A.A.A.; Kadir, H.A.; Tayyab, S. Ticari fisinin yapısal stabilitesine farklı denature edici koşulların etkisi. Turk. J. Biochem. 2013, 38, 319–328. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kwon, J.H.; Imran, M.; Sohaib, M.; Aslam, A.; Nawaz, I.; Amjad, Z.; Khan, U.; Javed, M. Plant and bacterial proteases: A key towards improving meat tenderization, a mini review. Cogent Food Agric. 2016, 2, 1261780. [Google Scholar] [CrossRef]
- Feijoo-Siota, L.; Villa, T.G. Native and Biotechnologically Engineered Plant Proteases with Industrial Applications. Food Bioprocess Technol. 2011, 4, 1066–1088. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Bezerra, C.S.; De Farias Lemos, C.M.G.; De Sousa, M.; Gonçalves, L.R.B. Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 132, 42125. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of Enzymes: A Literature Survey. Immobil. Enzym. Cells 2013, 1051, 15–31. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Motevalizadeh, S.F.; Khoobi, M.; Sadighi, A.; Khalilvand-Sedagheh, M.; Pazhouhandeh, M.; Ramazani, A.; Faramarzi, M.A.; Shafiee, A. Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. J. Mol. Catal. B Enzym. 2015, 120, 75–83. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Styevkó, G.; Ta, L.P.; Tran, A.T.M.; Bujna, E.; Orbán, P.; Dam, M.S.; Nguyen, Q.D. Immobilization and some properties of commercial enzyme preparation for production of lactulose-based oligosaccharides. Food Bioprod. Process. 2019, 107, 97–103. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- Gonçalves, M.C.P.; Kieckbusch, T.G.; Perna, R.F.; Fujimoto, J.T.; Morales, S.A.V.; Romanelli, J.P. Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochem. 2019, 76, 95–110. [Google Scholar] [CrossRef]
- Gu, Y.J.; Zhu, M.L.; Li, Y.L.; Xiong, C.H. Research of a new metal chelating carrier preparation and papain immobilization. Int. J. Biol. Macromol. 2018, 112, 1175–1182. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Saleh, S.A.A.; Abdel-Hameed, S.A.M.; Fayad, A.M. Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Santos, M.P.F.; Porfírio, M.C.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin immobilization: Influence of carbon support functionalization. Int. J. Biol. Macromol. 2022, 203, 67–79. [Google Scholar] [CrossRef]
- Santos, M.P.F.; Ferreira, M.A.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Functionalized activated carbon as support for trypsin immobilization and its application in casein hydrolysis. Bioprocess Biosyst. Eng. 2023, 46, 1651–1664. [Google Scholar] [CrossRef]
- Miguez, J.P.; Fernandez-Lafuente, R.; Tavano, O.L.; Mendes, A.A. The Immobilization and Stabilization of Trypsin from the Porcine Pancreas on Chitosan and Its Catalytic Performance in Protein Hydrolysis. Catalysts 2023, 13, 1344. [Google Scholar] [CrossRef]
- Demirkan, E.; Avci, T.; Aykut, Y. Protease immobilization on cellulose monoacetate/chitosan-blended nanofibers. J. Ind. Text. 2018, 47, 2092–2111. [Google Scholar] [CrossRef]
- Siar, E.H.; Zaak, H.; Kornecki, J.F.; Zidoune, M.N.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of ficin extract by immobilization on glyoxyl agarose. Preliminary characterization of the biocatalyst performance in hydrolysis of proteins. Process Biochem. 2017, 58, 98–104. [Google Scholar] [CrossRef]
- Husain, Q. Nanocarriers immobilized proteases and their industrial applications: An overview. J. Nanosci. Nanotechnol. 2018, 18, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent immobilization of proteases on polylactic acid for proteins hydrolysis and waste biomass protein content valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- Cesaretti, A.; Montegiove, N.; Calzoni, E.; Leonardi, L.; Emiliani, C. Protein Hydrolysates: From Agricultural Waste Biomasses To High Added-Value Products (Minireview). Agrolife Sci. J. 2020, 9, 79–87. [Google Scholar]
- Wei, X.; Liu, D.; Li, W.; Liao, L.; Wang, Z.; Huang, W.; Huang, W. Biochar addition for accelerating bioleaching of heavy metals from swine manure and reserving the nutrients. Sci. Total Environ. 2018, 631–632, 1553–1559. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Yang, G.; Lv, M.; Zhang, L. Covalent immobilization of alkaline proteinase on amino-functionalized magnetic nanoparticles and application in soy protein hydrolysis. Biotechnol. Prog. 2019, 35, e2756. [Google Scholar] [CrossRef]
- Wang, S.-N.; Zhang, C.-R.; Qi, B.-K.; Sui, X.-N.; Jiang, L.-Z.; Li, Y.; Wang, Z.-J.; Feng, H.-X.; Wang, R.; Zhang, Q.-Z. Immobilized alcalase alkaline protease on the magnetic chitosan nanoparticles used for soy protein isolate hydrolysis. Eur. Food Res. Technol. 2014, 239, 1051–1059. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, J.; Xing, L. Preparation of active corn peptides from zein through double enzymes immobilized with calcium alginate-chitosan beads. Process Biochem. 2014, 49, 1682–1690. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.-H.; Lou, W.-Y. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Coord. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Fadýloǧlu, S. Immobilization and characterization of ficin. Food/Nahrung 2001, 45, 143–146. [Google Scholar] [CrossRef]
- Metin, A.Ü.; Alver, E. Fibrous polymer-grafted chitosan/clay composite beads as a carrier for immobilization of papain and its usability for mercury elimination. Bioprocess Biosyst. Eng. 2016, 39, 1137–1149. [Google Scholar] [CrossRef]
- Homaei, A.; Samari, F. Investigation of activity and stability of papain by adsorption on multi-wall carbon nanotubes. Int. J. Biol. Macromol. 2017, 105, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wang, W.; Xue, C.; Mao, X. Effective Enzyme Immobilization onto a Magnetic Chitin Nanofiber Composite. ACS Sustain. Chem. Eng. 2018, 6, 8118–8124. [Google Scholar] [CrossRef]
- Mehdi, W.A.; Mehde, A.A.; Özacar, M.; Özacar, Z. Characterization and immobilization of protease and lipase on chitin-starch material as a novel matrix. Int. J. Biol. Macromol. 2018, 117, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.F.C.; Santos, M.P.F.; Barbosa, R.P.; Renata Bonomo, C.F.; Veloso, C.M.; Souza Júnior, E.C. Pepsin immobilization on activated carbon and functionalized with glutaraldehyde and genipin for the synthesis of antioxidant peptides of goat casein. Food Res. Int. 2024, 114161. [Google Scholar] [CrossRef]
- Daglioglu, C.; Zihnioglu, F. Covalent immobilization of trypsin on glutaraldehyde-activated silica for protein fragmentation. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 378–384. [Google Scholar] [CrossRef]
- Mosafa, L.; Moghadam, M.; Shahedi, M. Papain enzyme supported on magnetic nanoparticles: Preparation, characterization and application in the fruit juice clarification. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 1897–1904. [Google Scholar] [CrossRef]
- Zlateski, V.; Fuhrer, R.; Koehler, F.M.; Wharry, S.; Zeltner, M.; Stark, W.J.; Moody, T.S.; Grass, R.N. Efficient magnetic recycling of covalently attached enzymes on carbon-coated metallic nanomagnets. Bioconjugate Chem. 2014, 25, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Lakouraj, M.M.; Maashsani, A.; Norouzian, R.S.; Mohadjerani, M. Immobilization of pepsin on chitosan magnetic nanoparticles and its application in deacetylation of amides. J. Carbohydr. Chem. 2015, 34, 103–119. [Google Scholar] [CrossRef]
- Silva, T.R.; Rodrigues, D.P.; Rocha, J.M.S.S.; Gil, M.H.; Pinto, S.C.S.S.; Lopes-Da-Silva, J.A.; Guiomar, A.J. Immobilization of trypsin onto poly(ethylene terephthalate)/poly(lactic acid) nonwoven nanofiber mats. Biochem. Eng. J. 2015, 104, 48–56. [Google Scholar] [CrossRef]
- Silva, D.F.; Rosa, H.; Carvalho, A.F.A.; Oliva-Neto, P. Immobilization of papain on chitin and chitosan and recycling of soluble enzyme for deflocculation of Saccharomyces cerevisiae from bioethanol distilleries. Enzym. Res. 2015, 2015, 573721. [Google Scholar] [CrossRef] [PubMed]
- Zappino, M.; Cacciotti, I.; Benucci, I.; Nanni, F.; Liburdi, K.; Valentini, F.; Esti, M. Bromelain immobilization on microbial and animal source chitosan films, plasticized with glycerol, for application in wine-like medium: Microstructural, mechanical and catalytic characterisations. Food Hydrocoll. 2015, 45, 41–47. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Liu, Z.; Huang, H. Pineapple peel carboxymethyl cellulose/polyvinyl alcohol/mesoporous silica SBA-15 hydrogel composites for papain immobilization. Carbohydr. Polym. 2017, 169, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, B.; Shi, Y.; Yang, L.; Ma, H.L. Activity and Stability of Trypsin Immobilized onto Chitosan Magnetic Nanoparticles. Adv. Mater. Sci. Eng. 2017, 2017, 1457072. [Google Scholar] [CrossRef]
- Rojas-Mercado, A.S.; Moreno-Cortez, I.E.; Lucio-Porto, R.; Pavón, L.L. Encapsulation and immobilization of ficin extract in electrospun polymeric nanofibers. Int. J. Biol. Macromol. 2018, 118, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Xi, Y.; Zhang, L.; Ye, T.; Zhao, X. Enhanced activity and stability of papain by covalent immobilization on porous magnetic nanoparticles. Int. J. Biol. Macromol. 2018, 114, 143–148. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Immobilization/Stabilization of Ficin Extract on Glutaraldehyde-Activated Agarose Beads. Catalysts 2018, 8, 149. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S. Immobilization of trypsin from porcine pancreas onto chitosan nonwoven by covalent bonding. Polymers 2019, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Vallés, D.; Furtado, S.; Villadóniga, C.; Cantera, A.M.B. Adsorption onto alumina and stabilization of cysteine proteinases from crude extract of Solanum granuloso-leprosum fruits. Process Biochem. 2011, 46, 592–598. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; Guibaud, G.; Lens, P.N.L.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D. Lead sorption by biochar produced from digestates: Consequences of chemical modification and washing. J. Environ. Manag. 2018, 219, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Bijoy, G.; Rajeev, R.; Benny, L.; Jose, S.; Varghese, A. Enzyme immobilization on biomass-derived carbon materials as a sustainable approach towards environmental applications. Chemosphere 2022, 307, 135759. [Google Scholar] [CrossRef]
- Santos, M.P.F.; da Silva, J.F.; da Costa Ilhéu Fontan, R.; Bonomo, R.C.F.; Santos, L.S.; Veloso, C.M. New insight about the relationship between the main characteristics of precursor materials and activated carbon properties using multivariate analysis. Can. J. Chem. Eng. 2020, 98, 1501–1511. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.A.; Hossain, M.D.; Johir, M.A.H.; Hossen, J.; Rahman, M.S.; Zhou, J.L.; Hasan, A.T.; Karmakar, A.K.; Ahmed, M.B. The Potentiality of Rice Husk-Derived Activated Carbon: From Synthesis to Application. Processes 2020, 8, 203. [Google Scholar] [CrossRef]
- Fierro, V.; Muñiz, G.; Basta, A.H.; El-Saied, H.; Celzard, A. Rice straw as precursor of activated carbons: Activation with ortho-phosphoric acid. J. Hazard. Mater. 2010, 181, 27–34. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Bubanale, S.; Shivashankar, M. History, Method of Production, Structure and Applications of Activated Carbon. Int. J. Eng. Res. 2017, 6, 495–498. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption- and decomposition-based techniques for the regeneration of activated carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Frank, J.; Ruhl, A.S.; Jekel, M. Impacts of backwashing on granular activated carbon filters for advanced wastewater treatment. Water Res. 2015, 87, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crop. Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Bassan, J.C.; De Souza Bezerra, T.M.; Peixoto, G.; Zanutto, C.; Da Cruz, P.; Galán, J.P.M.; Vaz, A.B.D.S.; Garrido, S.S.; Filice, M.; Monti, R. Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein. Materials 2016, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Okura, N.S.; Sabi, G.J.; Crivellenti, M.C.; Gomes, R.A.B.; Fernandez-Lafuente, R.; Mendes, A.A. Improved immobilization of lipase from Thermomyces lanuginosus on a new chitosan-based heterofunctional support: Mixed ion exchange plus hydrophobic interactions. Int. J. Biol. Macromol. 2020, 163, 550–561. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed]
- Pego, M.F.F.; Bianchi, M.L.; Carvalho, J.A.; Veiga, T.R.L.A. Surface modification of activated carbon by corona treatment. Ann. Braz. Acad. Sci. 2019, 91, e20170947. [Google Scholar] [CrossRef]
- Du, J.R.; Hsu, L.H.; Xiao, E.S.; Guo, X.; Zhang, Y.; Feng, X. Using genipin as a “green” crosslinker to fabricate chitosan membranes for pervaporative dehydration of isopropanol. Sep. Purif. Technol. 2020, 244, 116843. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, L.; Bao, Y.; Hao, A.; Qin, Y.; Wen, Z.; Xiu, Z. Biotransformation of geniposide in Gardenia jasminoides to genipin by Trichoderma harzianum CGMCC 2979. Cuihua Xuebao/Chin. J. Catal. 2014, 35, 1534–1546. [Google Scholar] [CrossRef]
- Bellé, A.S.; Hackenhaar, C.R.; Spolidoro, L.S.; Rodrigues, E.; Klein, M.P.; Hertz, P.F. Efficient enzyme-assisted extraction of genipin from genipap (Genipa americana L.) and its application as a crosslinker for chitosan gels. Food Chem. 2018, 246, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Atta-Obeng, E.; Dawson-Andoh, B.; Seehra, M.S.; Geddam, U.; Poston, J.; Leisen, J. Physico-chemical characterization of carbons produced from technical lignin by sub-critical hydrothermal carbonization. Biomass Bioenergy 2017, 107, 172–181. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Niu, T.; Huang, R.; Li, S.; Sun, J.; Wang, Y. Facile synthesis of reusable magnetic Fe/Fe3C/C composites from renewable resources for super-fast removal of organic dyes: Characterization, mechanism and kinetics. Powder Technol. 2019, 351, 314–324. [Google Scholar] [CrossRef]
- Omar, A.H.; Muda, K.; Majid, Z.A.; Affam, A.C.; Ezechi, E.H. Effect of magnetic activated carbon on the surface hydrophobicity for initial biogranulation via response surface methodology. Water Environ. Res. 2020, 92, 73–83. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Sousa, E.Y.A.D.; Serpa, J.D.F.; Oliveira, R.C.; Santos, J.C.S.D. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quím. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Bresolin, I.T.L.; Miranda, E.A.; Bueno, S.M.A. Cromatografia de afinidade por íons metálicos imobilizados (IMAC) de biomoléculas: Aspectos fundamentais e aplicações tecnológicas. Quím. Nova 2009, 32, 1288–1296. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Zhang, H.; Li, Y.; Liu, Y.; Xu, L.; Wang, Y.; Fei, X.; Tian, J. A novel catalytic material for hydrolyzing cow’s milk allergenic proteins: Papain-Cu3(PO4)2·3H2O-magnetic nanoflowers. Food Chem. 2020, 311, 125911. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and characterization of a milk-clotting aspartic proteinase from globe artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef]
- Capobiango, M.; Lopes, D.C.F.; Carreira, R.L.; Afonso, W.D.O.; Segall, S.D.; Silvestre, M.P.C. Optimization of enzyme assisted processes for extracting and hydrolysing corn proteins aiming phenylalanine removal. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Swarnalatha, S.; Kamatchi, P.; Kirubagaran, R.; Perinmbam, K.; Sekaran, G. Immobilization of proteolytic enzyme on highly porous activated carbon derived from rice bran. J. Porous Mater. 2009, 16, 439–445. [Google Scholar] [CrossRef]
- Kumar, A.G.; Swarnalatha, S.; Kamatchi, P.; Sekaran, G. Immobilization of high catalytic acid protease on functionalized mesoporous activated carbon particles. Biochem. Eng. J. 2009, 43, 185–190. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Perinbam, K.; Kamatchi, P.; Nagesh, N.; Sekaran, G. In situ immobilization of acid protease on mesoporous activated carbon packed column for the production of protein hydrolysates. Bioresour. Technol. 2010, 101, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Armelin, E.; Moreno-Martínez, J.A.; Alemán, C.; Ferreira, C.A. Transport and antifouling properties of papain-based antifouling coatings. Appl. Surf. Sci. 2015, 341, 75–85. [Google Scholar] [CrossRef]
- Salleh, N.H.M.; Arbain, D.; Daud, M.Z.M.; Zainalabidin, N. Preparation and characterisation of rice husk activated carbon for neutral protease adsorption. Mater. Res. Innov. 2014, 18, S6-307–S6-309. [Google Scholar] [CrossRef]
- Pounsamy, M.; Somasundaram, S.; Palanivel, S.; Balasubramani, R.; Chang, S.W.; Nguyen, D.D.; Ganesan, S. A novel protease-immobilized carbon catalyst for the effective fragmentation of proteins in high-TDS wastewater generated in tanneries: Spectral and electrochemical studies. Environ. Res. 2019, 172, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Liu, J.Y.; Wang, X.Y.; Sun, B.G.; Zhao, Z.Y.; Pei, P.G.; Li, X.Y. Preparation of high fischer ratio oligopeptide of chlorella powder using specific enzymatic hydrolysis. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega. 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Morellon-Sterling, R.; Tavano, O.; Bolivar, J.M.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Sabir, J.S.M.; Tacias-Pascacio, V.G.; Fernandez-Lafuente, R. A review on the immobilization of pepsin: A Lys-poor enzyme that is unstable at alkaline pH values. Int. J. Biol. Macromol. 2022, 210, 682–702. [Google Scholar] [CrossRef]
- Luk, V.N.; Fiddes, L.K.; Luk, V.M.; Kumacheva, E.; Wheeler, A.R. Digital microfluidic hydrogel microreactors for proteomics. Proteomics 2012, 12, 1310–1318. [Google Scholar] [CrossRef]
- Motoi, H.; Fukudome, S.; Urabe, I. Continuous production of wheat gluten peptide with foaming properties using immobilized enzymes. Eur. Food Res. Technol. 2004, 219, 522–528. [Google Scholar] [CrossRef]
- Kumari, A.; Kaur, B.; Srivastava, R.; Sangwan, R.S. Isolation and immobilization of alkaline protease on mesoporous silica and mesoporous ZSM-5 zeolite materials for improved catalytic properties. Biochem. Biophys. Rep. 2015, 2, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Caso, M.C.; Bavaro, T.; Masci, S.; Keršienė, M.; Esti, M. Prolyl endopeptidase from Aspergillus niger immobilized on a food-grade carrier for the production of gluten-reduced beer. Food Control 2020, 110, 106987. [Google Scholar] [CrossRef]



| Support | Enzyme | Method | Results | References |
|---|---|---|---|---|
| Physical (Adsorption) | ||||
| Celite | Ficin | pH 7 for 10 min | Immobilized enzyme activity of 160 U/mg for casein hydrolysis | [80] |
| Activated carbon | Papain | pH 7.5 for 0.5 h | Immobilization capacity of 97 mg/g and enzyme activity of 75 mg Phe/100 g for whey hydrolysis | [17] |
| Activated carbon | Pancreatin | 30 min; 25 °C | Immobilized enzymes with 84% activity for the removal of phenylalanine | [17] |
| Polymer-modified chitosan/clay Composite | Papain | pH 7; 20 °C | Immobilization capacity of 34.47 mg/g and residual catalytic activity of 100% for BAEE hydrolysis | [81] |
| Multi walled carbon nanotubes | Papain | pH 7; 2 h; 200 rpm | Immobilization efficiency of 4.2 mg/mL with enzyme activity of 67% for casein hydrolysis | [82] |
| Magnetic Chitin Nanofiber Composite | α-chymotrypsin | 2 h; 20 °C; 200 rpm | Immobilization capacity of 92.4 mg/g with a relative activity of 100% for casein hydrolysis | [83] |
| Chitin | Proteases | pH 7.5; 4 °C overnight | Recovered enzyme activity of 2.5% for casein hydrolysis | [84] |
| Activated carbon | Pepsin | pH 3; 2 h, 30 rpm | Immobilization efficiency of 93.6% with enzyme activity of 1.3 U·mg−1 for hydrolysis of bovine casein | [10] |
| Activated carbon | Trypsin | pH 8; 2 h, 30 rpm | Immobilization efficiency of 87.5% with enzyme activity of 2.5 U·mg −1 for hydrolysis of goat casein | [5] |
| Activated carbon | Trypsin | pH 5, 30 rpm, 2 h | Immobilization efficiency of over 91% with enzymatic activity of 2.60 U for casein hydrolysis | [64] |
| Chitosan | Trypsin | pH 9, 200 rpm, 12 h | Immobilization efficiency of over 19% with enzymatic activity of 21.1 nmol·min−1·mg−1 for BSA hydrolysis | [65] |
| Activated carbon | Pepsin | pH 3, 30 rpm, 2 h | Immobilization efficiency of 98% with enzymatic activity of 0.95 U for casein hydrolysis | [85] |
| Chemical (Covalent bonding) | ||||
| Glutaraldehyde-activated silica | Trypsin | pH 7.5, 4 °C, 1 h, 200 rpm | Immobilization efficiency of 63% with enzyme activity of 92 nmol/min/mg for BSA hydrolysis | [86] |
| Silica-coated Fe3O4 nanoparticles | Papain | pH 7.5, 2 h | Immobilization efficiency of 57.9% with enzyme activity of 86% for hydrolysis of bovine casein | [87] |
| Carbon coated nanoparticles | α-chymotrypsin | - | Immobilization capacity of 50 mg/g with 25% hydrolysis activity of N-benzoyl-L-tyrosine ethyl ester substrate | [88] |
| Magnetic chitosan nanoparticles | Pepsin | - | Immobilization capacity of 99 mg/g with enzyme activity of 85% for amide hydrolysis | [89] |
| Poly (ethylene terephthalate) (PET) with PVA | Trypsin | pH 5.5, 2 h | Immobilization capacity of 0.62 µmol pNA min−1g−1 mat for BAPNA hydrolysis | [90] |
| Glutaraldehyde-modified chitosan | Papain | pH 8, 5 h | Enzyme activity of 2.7 U/g for hydrolysis of azocasein sulfanilamide | [91] |
| Glutaraldehyde-modified chitosan | Stem Bromelin | pH 3.2, 150 rpm, 20 °C overnight | Immobilization efficiency of 41% | [92] |
| Pineapple Peel Carboxymethyl Cellulose (PCMC)/Polyvinyl Alcohol (PVA)/Mesoporous Silica SBA-15 hydrogel composites | Papain | pH 6.5, 1.5 h | Immobilization capacity of the hydrogel of 100% with enzyme activity of 1800 U/g for casein hydrolysis | [93] |
| Glyoxyl-agarose support | Ficin | pH 10; 25 °C, 3 h | Immobilization efficiency of 100% and relative activity of 40% for the hydrolysis of Benzoyl-arginine-p-nitroanilide (BANA) | [67] |
| Magnetic chitosan nanoparticles | Trypsin | pH 7.5, 25 °C, 1 h, 200 rpm | Immobilization capacity of 149.25 mg/g with residual activity of 100% for BAEE hydrolysis | [94] |
| Magnetic Chitin Nanofiber Composite | α-chymotrypsin | 20 °C, 2 h, 200 rpm | Immobilization capacity of 581.84 mg/g with a relative activity of 100% for casein hydrolysis | [83] |
| Electrospun PVA Nanofibers | Ficin | pH 8, 1 h | Immobilization capacity of 92% for hydrolysis of Nα-benzoyl-L-arginine 4-nitroanilide hydrochloride (BAPA) | [95] |
| Porous magnetic nanoparticles | Papain | 25 °C, 12 h. | Immobilization efficiency of 82% with a casein hydrolysis capacity of 4.95 mg/L·min | [96] |
| Glutaraldehyde-activated agarose beads | Ficin | pH 7, 25 °C, 4 h | Immobilization efficiency of 100% with enzyme activity of 40% for casein hydrolysis | [97] |
| Glutaraldehyde-Modified Chitin | Protease from sunflower seeds | pH 7.5, 4 °C, 12 h | Recovered enzyme activity of 38% for casein hydrolysis | [84] |
| Glutaraldehyde-Modified Chitin | Trypsin | pH 8.5, 25 °C, 30 min | Relative activity of 100% for hydrolysis of Nα-benzoyl-L-arginine 4-nitroanilide hydrochloride (L-BAPA) | [98] |
| Glutaraldehyde-Modified Activated carbon | Pepsin | pH 3, 30 rpm, 2 h | Immobilization efficiency of 94.9% with enzyme activity of 1.75 U·mg −1 for the hydrolysis of bovine casein | [10] |
| Glutaraldehyde-Modified Activated carbon | Trypsin | pH 8, 30 rpm, 2 h | Immobilization efficiency of 91% with enzyme activity of 3 U·mg −1 for goat casein hydrolysis | [5] |
| Activated carbon modified with metal ions | Trypsin | pH 5, 30 rpm, 2 h | Immobilization efficiency of over 95% with enzymatic activity of 4.11 U for casein hydrolysis | [64] |
| Glutaraldehyde–glycine activated chitosan | Trypsin | pH 9, 200 rpm, 12 h | Immobilization efficiency of over 81% with enzymatic activity of 33.1 nmol·min−1·mg−1 for BSA hydrolysis | [65] |
| Activated carbon modified with genipin | Pepsin | pH 3, 30 rpm, 2 h | Immobilization efficiency of 98% with enzymatic activity of 1.39 U for casein hydrolysis | [85] |
| Method | Application | Activity Free Enzyme | Activity Immobilized Enzyme | References |
|---|---|---|---|---|
| Pepsin | ||||
| Adsorption | Hydrolysis of bovine casein | 41.67 U | 245.02 U—8 cycles | [10] |
| Covalent bonding | 299.79 U—8 cycles | |||
| Adsorption | Hydrolysis of bovine casein | 3.32 U | 1.04 U—1 cycle | [63] |
| Covalent bonding (glutaraldehyde) | 1.10 U—1 cycle | |||
| Covalent bonding (genipin) | 1.84 U—1 cycle | |||
| Covalent bonding (metal ions) | 2.30 U—1 cycle | |||
| Adsorption | Hydrolysis of goat casein | 2.90 U | 4.35 U—8 cycles | [85] |
| Covalent bonding (glutaraldehyde) | 3.50 U—8 cycles | |||
| Covalent bonding (genipin) | 6.35 U—8 cycles | |||
| Trypsin | ||||
| Adsorption | Hydrolysis of goat casein, among others | 3.35 U | 9.22 U—4 cycles | [5] |
| Covalent bonding | 10.45 U—4 cycles | |||
| Adsorption | Hydrolysis of bovine casein | 3.76 U | 3.30 U—2 cycles | [64] |
| Covalent bonding (glutaraldehyde) | 3.20 U—2 cycles | |||
| Covalent bonding (genipin) | 5.45 U—4 cycles | |||
| Covalent bonding (metal ions) | 16.74 U—6 cycles | |||
| Activated Carbon | Modification | Enzyme | References |
|---|---|---|---|
| Commercial | Copper Phosphate Magnetization | Papain | [125] |
| From pupunha palm | Modification with glutaraldehyde | Pepsin | [10] |
| From yellow mombin fruit stones | Modification with glutaraldehyde | Trypsin | [5] |
| From tamarind seeds | Modification with: Glutaraldehyde; Genipin; iron salts (Fe2+ and Fe3+) | Pepsin | [63] |
| From tamarind seeds | Modification with: Glutaraldehyde; Genipin; iron salts (Fe2+ and Fe3+) | Trypsin | [64] |
| From Umbu seeds | Modification with: Glutaraldehyde; Genipin | Pepsin | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.P.F.; de Souza Junior, E.C.; Villadóniga, C.; Vallés, D.; Castro-Sowinski, S.; Bonomo, R.C.F.; Veloso, C.M. Proteases: Importance, Immobilization Protocols, Potential of Activated Carbon as Support, and the Importance of Modifying Supports for Immobilization. BioTech 2024, 13, 13. https://doi.org/10.3390/biotech13020013
Santos MPF, de Souza Junior EC, Villadóniga C, Vallés D, Castro-Sowinski S, Bonomo RCF, Veloso CM. Proteases: Importance, Immobilization Protocols, Potential of Activated Carbon as Support, and the Importance of Modifying Supports for Immobilization. BioTech. 2024; 13(2):13. https://doi.org/10.3390/biotech13020013
Chicago/Turabian StyleSantos, Mateus Pereira Flores, Evaldo Cardozo de Souza Junior, Carolina Villadóniga, Diego Vallés, Susana Castro-Sowinski, Renata Cristina Ferreira Bonomo, and Cristiane Martins Veloso. 2024. "Proteases: Importance, Immobilization Protocols, Potential of Activated Carbon as Support, and the Importance of Modifying Supports for Immobilization" BioTech 13, no. 2: 13. https://doi.org/10.3390/biotech13020013