Synthesis and Characterization of Lignin-Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lignin Solubility
2.2. Approptiate Conditions for the Preparation of Lig-AgNPs
2.3. Core and Surface Characterisation of Lig-AgNPs
3. Materials and Methods
3.1. Lignin Isolation
3.2. Lignin Dissolution
3.3. Preparation and Separation of Lig-AgNPs
3.4. Characterization of Lig-AgNPs by UV-Vis
3.5. Determination of Silver Mass Concentration by AAS
3.6. DLS Analysis and Zeta Potential Determination
3.7. TEM Analysis
3.8. Lignin and Lig-AgNPs Characterization by FTIR
3.9. Characterization of Lig-AgNPs by XRD
3.10. Characterization of LigNPs by XPS
3.11. Photothermal Effect
4. Conclusions
Supplementary Materials
 lignin;
lignin;  unseparated (P+S) Lig-AgNPs;
unseparated (P+S) Lig-AgNPs;  separated (P) Lig-AgNPs;
separated (P) Lig-AgNPs;  supernatant (S). Figure S2: Samples of unseparated (P+S) Lignin–Ag-NPs prepared in a total volume of 8.4 mL, UPW, pH 11.00 ± 0.05. Mass reaction ratios mAg:mLig increase from sample 1 to sample 12. (a) Initial lignin concentration of 5.0 g L−1; (b) initial lignin concentration of 10.0 g L−1. Figure S3: Microscopy revealing the presence of a lignin envelope surrounding the silver core of Lig-AgNPs: (a) transmission electron microscopy (TEM); (b) scanning electron microscopy (SEM).
supernatant (S). Figure S2: Samples of unseparated (P+S) Lignin–Ag-NPs prepared in a total volume of 8.4 mL, UPW, pH 11.00 ± 0.05. Mass reaction ratios mAg:mLig increase from sample 1 to sample 12. (a) Initial lignin concentration of 5.0 g L−1; (b) initial lignin concentration of 10.0 g L−1. Figure S3: Microscopy revealing the presence of a lignin envelope surrounding the silver core of Lig-AgNPs: (a) transmission electron microscopy (TEM); (b) scanning electron microscopy (SEM).Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maťátková, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.H.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Ho, W.-J.; Fen, S.-K.; Liu, J.-J. Plasmonic effects of silver nanoparticles with various dimensions embedded and non-embedded in silicon dioxide antireflective coating on silicon solar cells. Appl. Phys. A 2017, 124, 29. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kneipp, K. Size-Dependent Shifts of Plasmon Resonance in Silver Nanoparticle Films Using Controlled Dissolution: Monitoring the Onset of Surface Screening Effects. J. Phys. Chem. C 2014, 118, 28075–28083. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, D.; Okram, G.S. Influence of surfactant, particle size and dispersion medium on surface plasmon resonance of silver nanoparticles. J. Phys. Condens. Matter 2020, 32, 145302. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the importance of aging, environment, size and stabilization mechanisms on the stability of metal nanoparticles: A case study for silver nanoparticles in a minimally defined and complex undefined bacterial growth medium. Nanomaterials 2019, 9, 1684. [Google Scholar] [CrossRef]
- Takesue, M.; Tomura, T.; Yamada, M.; Hata, K.; Kuwamoto, S.; Yonezawa, T. Size of Elementary Clusters and Process Period in Silver Nanoparticle Formation. J. Am. Chem. Soc. 2011, 133, 14164–14167. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Shankar, S.; Rhim, J.-W. Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 2017, 71, 76–84. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, X.; Liu, Z.; Li, Y. Facile and Efficient Synthesis of Silver Nanoparticles Based on Biorefinery Wood Lignin and Its Application as the Optical Sensor. ACS Sustain. Chem. Eng. 2018, 6, 7695–7703. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef]
- Nishimura, S.; Mott, D.; Takagaki, A.; Maenosono, S.; Ebitani, K. Role of base in the formation of silver nanoparticles synthesized using sodium acrylate as a dual reducing and encapsulating agent. Phys. Chem. Chem. Phys. 2011, 13, 9335–9343. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Hyeon, T. Formation Mechanisms of Uniform Nanocrystals via Hot-Injection and Heat-Up Methods. Small 2011, 7, 2685–2702. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Serra, A.; Filippo, E.; Re, M.; Palmisano, M.; Vittori-Antisari, M.; Buccolieri, A.; Manno, D. Non-functionalized silver nanoparticles for a localized surface plasmon resonance-based glucose sensor. Nanotechnology 2009, 20, 165501. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Božanić, D.K.; Bibić, N.; Šaponjić, Z.V.; Nedeljković, J.M. Optical properties of shaped silver nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 3511–3515. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer Blending as a Novel Approach for Tuning the SPR Peaks of Silver Nanoparticles. Polymers 2017, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Hollertz, R.; Arwin, H.; Faure, B.; Zhang, Y.; Bergström, L.; Wågberg, L. Dielectric properties of lignin and glucomannan as determined by spectroscopic ellipsometry and Lifshitz estimates of non-retarded Hamaker constants. Cellulose 2013, 20, 1639–1648. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nucleation. Cryst. Growth Des. 2010, 10, 5007–5019. [Google Scholar] [CrossRef]
- Hatami, N.; Ghader, S. Induction time of silver nanoparticles precipitation: Experiment and modeling. Cryst. Res. Technol. 2009, 44, 953–960. [Google Scholar] [CrossRef]
- Ghader, S.; Manteghian, M.; Kokabi, M.; Mamoory, R.S. Induction Time of Reaction Crystallization of Silver Nanoparticles. Chem. Eng. Technol. 2007, 30, 1129–1133. [Google Scholar] [CrossRef]
- Schall, J.M.; Capellades, G.; Myerson, A.S. Methods for estimating supersaturation in antisolvent crystallization systems. CrystEngComm 2019, 21, 5811–5817. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of Colloidal Silver Nanoparticles: Capping Action of Citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Wang, W.; Gu, B. Preparation and Characterization of Silver Nanoparticles at High Concentrations. In Concentrated Dispersions; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 878, pp. 1–14. [Google Scholar]
- Pal, T.; Sau, T.K.; Jana, N.R. Reversible Formation and Dissolution of Silver Nanoparticles in Aqueous Surfactant Media. Langmuir 1997, 13, 1481–1485. [Google Scholar] [CrossRef]
- Cai, Y.; Tan, F.; Qiao, X.; Wang, W.; Chen, J.; Qiu, X. Room-temperature synthesis of silica supported silver nanoparticles in basic ethanol solution and their antibacterial activity. RSC Adv. 2016, 6, 18407–18412. [Google Scholar] [CrossRef]
- Murray, B.J.; Li, Q.; Newberg, J.T.; Menke, E.J.; Hemminger, J.C.; Penner, R.M. Shape- and Size-Selective Electrochemical Synthesis of Dispersed Silver(I) Oxide Colloids. Nano Lett. 2005, 5, 2319–2324. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Mills, G.; Hajek, B. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. 1993, 97, 11542–11550. [Google Scholar] [CrossRef]
- Nayak, G.; Trivedi, M.K.; Branton, A.; Trivedi, D.; Jana, S. The physicochemical and thermal properties of consciousness energy healing treated silver oxide (Ag2O). Asp. Min. Miner. Sci. 2018, 2, 1–6. [Google Scholar]
- Dürauer, A.; Hobiger, S.; Walther, C.; Jungbauer, A. Mixing at the microscale: Power input in shaken microtiter plates. Biotechnol. J. 2016, 11, 1539–1549. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Du, A.; Zhang, Z.; Gao, G.; Yang, H.; Wu, J. Facile synthesis of silver nanoparticles with high concentration via a CTAB-induced silver mirror reaction. Colloids Surf. A Physicochem. Eng. Asp. 2012, 400, 73–79. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Santos, P.S.B.D.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kuga, S.; Okano, T. Mechanism of mercerization revealed by X-ray diffraction. J. Wood Sci. 2000, 46, 452–457. [Google Scholar] [CrossRef]
- Kamida, K.; Okajima, K.; Matsui, T.; Kowsaka, K. Study on the Solubility of Cellulose in Aqueous Alkali Solution by Deuteration IR and 13C NMR. Polym. J. 1984, 16, 857–866. [Google Scholar] [CrossRef]
- Le Moigne, N.; Navard, P. Dissolution mechanisms of wood cellulose fibres in NaOH–water. Cellulose 2010, 17, 31–45. [Google Scholar] [CrossRef]
- Miškovská, A.; Michailidu, J.; Kolouchová, I.J.; Barone, L.; Gornati, R.; Montali, A.; Tettamanti, G.; Berini, F.; Marinelli, F.; Masák, J.; et al. Biological activity of silver nanoparticles synthesized using viticultural waste. Microb. Pathog. 2024, 190, 106613. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Abranches, D.O.; da Costa Lopes, A.M.; Coutinho, J.A.P.; da Costa, M.C. Kraft Lignin Solubility and Its Chemical Modification in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18577–18589. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhu, M.-F. A comparison of the surface properties of lignin and sulfonated lignins by FTIR spectroscopy and wicking technique. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 57–60. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. A “Fourier Transformed Infrared” Compound Study of Lignin Recovered from a Formic Acid Process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Volkov, V.V.; Nuti, F.; Takaoka, Y.; Chelli, R.; Papini, A.M.; Righini, R. Hydration and Hydrogen Bonding of Carbonyls in Dimyristoyl-Phosphatidylcholine Bilayer. J. Am. Chem. Soc. 2006, 128, 9466–9471. [Google Scholar] [CrossRef]
- Petridis, L.; Smith, J.C. Conformations of Low-Molecular-Weight Lignin Polymers in Water. ChemSusChem 2016, 9, 289–295. [Google Scholar] [CrossRef]
- Westereng, B.; Cannella, D.; Wittrup Agger, J.; Jørgensen, H.; Larsen Andersen, M.; Eijsink, V.G.H.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5, 18561. [Google Scholar] [CrossRef]
- Kaur, R.; Ahluwalia, G.K.; Bakshi, M.S. Lignin-Induced Click Synthesis of Au, Ag, Pd, and Iron Oxide Nanoparticles and Their Nanocomposites in Aqueous Bulk and at the Solid–Liquid Interface. ACS Sustain. Chem. Eng. 2023, 11, 11819–11833. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.-K.; Kadam, A.; Kumar, G.; Jeon, B.-H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Si, M.; Yan, X.; Liu, M.; Shi, M.; Wang, Z.; Wang, S.; Zhang, J.; Gao, C.; Chai, L.; Shi, Y. In Situ Lignin Bioconversion Promotes Complete Carbohydrate Conversion of Rice Straw by Cupriavidus basilensis B-8. ACS Sustain. Chem. Eng. 2018, 6, 7969–7978. [Google Scholar] [CrossRef]
- Tian, G.; Zhong, X.; Wu, X.; Wang, Z. Self-Assembly Preparation of Nano-Lignin/Cationic Polyacrylamide Complexes. Polymers 2021, 13, 1726. [Google Scholar] [CrossRef]
- Ratnaweera, D.R.; Saha, D.; Pingali, S.V.; Labbé, N.; Naskar, A.K.; Dadmun, M. The impact of lignin source on its self-assembly in solution. RSC Adv. 2015, 5, 67258–67266. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Y.; Li, L.; Qiu, X. Atomic Force Microscopy and Molecular Dynamics Simulations for Study of Lignin Solution Self-Assembly Mechanisms in Organic–Aqueous Solvent Mixtures. ChemSusChem 2020, 13, 4420–4427. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, W.; Cao, J. Preparation of sodium ligninsulfonate-layered double hydroxide and its effects on wood flour/polypropylene composites during accelerated UV weathering. Polym. Compos. 2018, 39, 2451–2460. [Google Scholar] [CrossRef]
- Collier, W.; Kalasinsky, V.F.; Schultz, T.P. Infrared study of lignin: Assignment of methoxyl CH bending and stretching bands. Holzforschung 1997, 51, 167–168. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Ran, F.; Li, C.; Hao, Z.; Zhang, X.; Dai, L.; Si, C.; Shen, Z.; Qiu, Z.; Wang, J. Combined bactericidal process of lignin and silver in a hybrid nanoparticle on E. coli. Adv. Compos. Hybrid Mater. 2022, 5, 1841–1851. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; Botelho do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: An overview and comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef]
- Zook, J.M.; Long, S.E.; Cleveland, D.; Geronimo, C.L.A.; MacCuspie, R.I. Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV–visible absorbance. Anal. Bioanal. Chem. 2011, 401, 1993–2002. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef]
- Fan, M.; Thompson, M.; Andrade, M.L.; Brolo, A.G. Silver Nanoparticles on a Plastic Platform for Localized Surface Plasmon Resonance Biosensing. Anal. Chem. 2010, 82, 6350–6352. [Google Scholar] [CrossRef]
- Haes, A.J.; Duyne, R.P.V. Preliminary studies and potential applications of localized surface plasmon resonance spectroscopy in medical diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 527–537. [Google Scholar] [CrossRef]
- Singh, A.; Hou, W.-C.; Lin, T.-F.; Zepp, R.G. Roles of Silver–Chloride Complexations in Sunlight-Driven Formation of Silver Nanoparticles. Environ. Sci. Technol. 2019, 53, 11162–11169. [Google Scholar] [CrossRef]
- Rong, H.; Garg, S.; Waite, T.D. Transformation of AgCl Particles under Conditions Typical of Natural Waters: Implications for Oxidant Generation. Environ. Sci. Technol. 2018, 52, 11621–11631. [Google Scholar] [CrossRef]
- Hoyo, J.; Ivanova, K.; Torrent-Burgues, J.; Tzanov, T. Interaction of Silver-Lignin Nanoparticles with Mammalian Mimetic Membranes. Front. Bioeng. Biotechnol. 2020, 8, 439. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef]
- Zhang, S.; Du, C.; Wang, Z.; Han, X.; Zhang, K.; Liu, L. Reduced cytotoxicity of silver ions to mammalian cells at high concentration due to the formation of silver chloride. Toxicol. Vitr. 2013, 27, 739–744. [Google Scholar] [CrossRef]
- Zheng, P.; Xiang, L.; Chang, J.; Lin, Q.; Xie, L.; Lan, T.; Liu, J.; Gong, Z.; Tang, T.; Shuai, L.; et al. Nanomechanics of Lignin–Cellulase Interactions in Aqueous Solutions. Biomacromolecules 2021, 22, 2033–2042. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.D.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Merkl, P.; Zhou, S.; Zaganiaris, A.; Shahata, M.; Eleftheraki, A.; Thersleff, T.; Sotiriou, G.A. Plasmonic Coupling in Silver Nanoparticle Aggregates and Their Polymer Composite Films for Near-Infrared Photothermal Biofilm Eradication. ACS Appl. Nano Mater. 2021, 4, 5330–5339. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Delgado Vellosillo, I.; Lange, H.; Rova, U.; Christakopoulos, P.; Matsakas, L. Furan Distribution as a Severity Indicator upon Organosolv Fractionation of Hardwood Sawdust through a Novel Ternary Solvent System. ACS Sustain. Chem. Eng. 2024, 12, 1666–1680. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Particle shape: A review and new methods of characterization and classification. Sedimentology 2008, 55, 31–63. [Google Scholar] [CrossRef]
 1.00 g L−1,
1.00 g L−1,  2.50 g L−1,
2.50 g L−1,  5.00 g L−1,
5.00 g L−1,  10.00 g L−1, and
10.00 g L−1, and  20.00 g L−1, in a final volume of 210 µL. (a) Water, pH 11.00; (b) 60% (v/v) aqueous ethanol solution, pH 11. The red lines are the results of a linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
20.00 g L−1, in a final volume of 210 µL. (a) Water, pH 11.00; (b) 60% (v/v) aqueous ethanol solution, pH 11. The red lines are the results of a linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
 1.00 g L−1,
1.00 g L−1,  2.50 g L−1,
2.50 g L−1,  5.00 g L−1,
5.00 g L−1,  10.00 g L−1, and
10.00 g L−1, and  20.00 g L−1, in a final volume of 210 µL. (a) Water, pH 11.00; (b) 60% (v/v) aqueous ethanol solution, pH 11. The red lines are the results of a linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
20.00 g L−1, in a final volume of 210 µL. (a) Water, pH 11.00; (b) 60% (v/v) aqueous ethanol solution, pH 11. The red lines are the results of a linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.


 unseparated (P+S) and
unseparated (P+S) and 
 separated (P) Lig-AgNPs on the reaction mass ratio between Ag+ and lignin in final volume of 8.4 mL. (a) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (b) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red lines are the results of linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs. Dashed lines represent the point where visually observed aggregates form. (c,d) The mass concentration of Ag+ in
separated (P) Lig-AgNPs on the reaction mass ratio between Ag+ and lignin in final volume of 8.4 mL. (a) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (b) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red lines are the results of linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs. Dashed lines represent the point where visually observed aggregates form. (c,d) The mass concentration of Ag+ in 
 unseparated (P+S) and
unseparated (P+S) and 
 separated (P) Lig-AgNPs and in
separated (P) Lig-AgNPs and in 
 supernatant (S). (c) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (d) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red dashed lines represent the calculated maximum mass concentration of Ag+.
supernatant (S). (c) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (d) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red dashed lines represent the calculated maximum mass concentration of Ag+.

 unseparated (P+S) and
unseparated (P+S) and 
 separated (P) Lig-AgNPs on the reaction mass ratio between Ag+ and lignin in final volume of 8.4 mL. (a) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (b) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red lines are the results of linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs. Dashed lines represent the point where visually observed aggregates form. (c,d) The mass concentration of Ag+ in
separated (P) Lig-AgNPs on the reaction mass ratio between Ag+ and lignin in final volume of 8.4 mL. (a) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (b) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red lines are the results of linear regression and include points with R-square (COD) ≥ 0.95. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs. Dashed lines represent the point where visually observed aggregates form. (c,d) The mass concentration of Ag+ in 
 unseparated (P+S) and
unseparated (P+S) and 
 separated (P) Lig-AgNPs and in
separated (P) Lig-AgNPs and in 
 supernatant (S). (c) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (d) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red dashed lines represent the calculated maximum mass concentration of Ag+.
supernatant (S). (c) Water, pH 11.00, initial lignin concentration 5.0 g L−1; (d) water, pH 11.00, initial lignin concentration 10.0 g L−1. The red dashed lines represent the calculated maximum mass concentration of Ag+.
 initial lignin concentration 5.0 g L−1;
initial lignin concentration 5.0 g L−1;  initial lignin concentration 10.0 g L−1. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
initial lignin concentration 10.0 g L−1. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
 initial lignin concentration 5.0 g L−1;
initial lignin concentration 5.0 g L−1;  initial lignin concentration 10.0 g L−1. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
initial lignin concentration 10.0 g L−1. Colored areas represent standard deviation values of 3 independently prepared batches of Lig-AgNPs.
 (c) 8.4 mL, 5.0 g L−1, 0.37 mAg+: mlig;
(c) 8.4 mL, 5.0 g L−1, 0.37 mAg+: mlig;  (d) 8.4 mL, 10.0 g L−1, 0.25 mAg+: mlig;
(d) 8.4 mL, 10.0 g L−1, 0.25 mAg+: mlig;  (e) 84.0 mL, 5.0 g L−1, 0.37 mAg+: mlig;
(e) 84.0 mL, 5.0 g L−1, 0.37 mAg+: mlig;  (f) 84.0 mL, 10.0 g L−1, 0.25 mAg+: mlig.
(f) 84.0 mL, 10.0 g L−1, 0.25 mAg+: mlig.
 (c) 8.4 mL, 5.0 g L−1, 0.37 mAg+: mlig;
(c) 8.4 mL, 5.0 g L−1, 0.37 mAg+: mlig;  (d) 8.4 mL, 10.0 g L−1, 0.25 mAg+: mlig;
(d) 8.4 mL, 10.0 g L−1, 0.25 mAg+: mlig;  (e) 84.0 mL, 5.0 g L−1, 0.37 mAg+: mlig;
(e) 84.0 mL, 5.0 g L−1, 0.37 mAg+: mlig;  (f) 84.0 mL, 10.0 g L−1, 0.25 mAg+: mlig.
(f) 84.0 mL, 10.0 g L−1, 0.25 mAg+: mlig.

 raw lignin; (b) lignin after NaOH treatment to pH 11, initial lignin concentrations
raw lignin; (b) lignin after NaOH treatment to pH 11, initial lignin concentrations  5.0 g L−1 and
5.0 g L−1 and  10.0 g L−1; (c) supernatant (S) after Lig-AgNPs separation, initial lignin concentrations
10.0 g L−1; (c) supernatant (S) after Lig-AgNPs separation, initial lignin concentrations  5.0 g L−1 and
5.0 g L−1 and  10.0 g L−1; (d) separated (P) Lig-AgNPs synthesized in a total volume of 84.0 mL, water, pH 11.00,
10.0 g L−1; (d) separated (P) Lig-AgNPs synthesized in a total volume of 84.0 mL, water, pH 11.00,  initial lignin concentration 5.0 g L−1 and reaction ratio 0.37 mAg:mLig and
initial lignin concentration 5.0 g L−1 and reaction ratio 0.37 mAg:mLig and  initial lignin concentration 10.0 g L−1 and reaction ratio 0.25 mAg:mLig.
initial lignin concentration 10.0 g L−1 and reaction ratio 0.25 mAg:mLig.
 raw lignin; (b) lignin after NaOH treatment to pH 11, initial lignin concentrations
raw lignin; (b) lignin after NaOH treatment to pH 11, initial lignin concentrations  5.0 g L−1 and
5.0 g L−1 and  10.0 g L−1; (c) supernatant (S) after Lig-AgNPs separation, initial lignin concentrations
10.0 g L−1; (c) supernatant (S) after Lig-AgNPs separation, initial lignin concentrations  5.0 g L−1 and
5.0 g L−1 and  10.0 g L−1; (d) separated (P) Lig-AgNPs synthesized in a total volume of 84.0 mL, water, pH 11.00,
10.0 g L−1; (d) separated (P) Lig-AgNPs synthesized in a total volume of 84.0 mL, water, pH 11.00,  initial lignin concentration 5.0 g L−1 and reaction ratio 0.37 mAg:mLig and
initial lignin concentration 5.0 g L−1 and reaction ratio 0.37 mAg:mLig and  initial lignin concentration 10.0 g L−1 and reaction ratio 0.25 mAg:mLig.
initial lignin concentration 10.0 g L−1 and reaction ratio 0.25 mAg:mLig.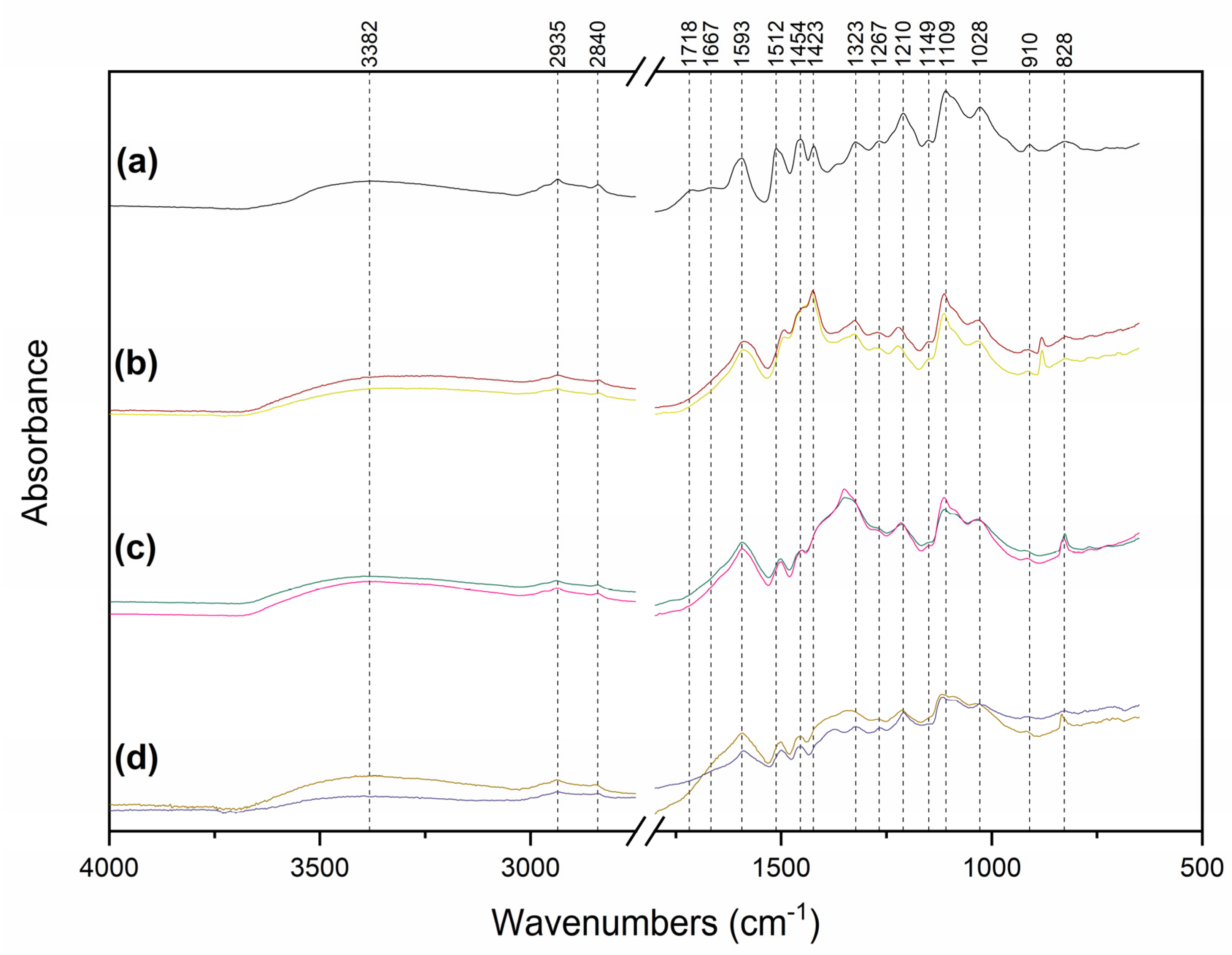


| Input Lig [g L−1] | pH 10 | pH 11 | pH 12 | |||
|---|---|---|---|---|---|---|
| Lignin [g L−1] | Dissolution [%] | Lignin [g L−1] | Dissolution [%] | Lignin [g L−1] | Dissolution [%] | |
| 1.00 | 0.52 ± 0.03 | 52 ± 3 | 0.88 ± 0.01 | 88 ± 1 | ~1.00 | ~100.0 |
| 2.50 | 1.05 ± 0.07 | 42 ± 3 | 1.99 ± 0.02 | 80 ± 1 | ~2.50 | ~100.0 |
| 5.00 | 1.91 ± 0.12 | 38 ± 2 | 4.12 ± 0.14 | 82 ± 3 | ~5.00 | ~100.0 |
| 10.00 | 3.56 ± 0.37 | 36 ± 4 | 8.05 ± 0.26 | 80 ± 3 | ~10.00 | ~100.0 |
| 20.00 | 6.64 ± 0.12 | 33 ± 1 | 14.02 ± 0.71 | 70 ± 4 | ~20.00 | ~100.0 |
| Reaction Medium | Initial Lignin Conc. [g L−1] | Slope (k) | Intercept (q) | R-Square (COD) |
|---|---|---|---|---|
| Water | 1.047 ± 0.065 | 0.023 ± 0.019 | 0.967 | |
| 2.50 | 2.762 ± 0.151 | 0.078 ± 0.036 | 0.977 | |
| 5.00 | 5.965 ± 0.285 | 0.231 ± 0.051 | 0.984 | |
| 10.00 | 12.47 ± 1.171 | 0.436 ± 0.163 | 0.950 | |
| 20.00 | 17.595 ± 1.337 | 0.681 ± 0.214 | 0.967 | |
| 60% (v/v) Ethanol | 1.00 | 1.864 ± 0.161 | 0.026 ± 0.015 | 0.964 |
| 2.50 | 3.883 ± 0.421 | 0.114 ± 0.032 | 0.955 | |
| 5.00 | 6.267 ± 0.565 | 0.285 ± 0.063 | 0.953 | |
| 10.00 | 9.857 ± 0.857 | 0.439 ± 0.079 | 0.964 | |
| 20.00 | 19.047 ± 2.307 | 0.841 ± 0.145 | 0.958 |
| Reaction Medium | Initial Lignin Conc. [g L−1] | Slope (k) | Intercept (q) | R-Square (COD) |
|---|---|---|---|---|
| Water | 5.00 (P+S) | 6.767 ± 0.100 | 0.244 ± 0.023 | 0.998 |
| 5.00 (P) | 7.174 ± 0.069 | 0.016 ± 0.016 | 0.999 | |
| 10.00 (P+S) | 12.153 ± 0.312 | 0.540 ± 0.057 | 0.995 | |
| 10.00 (P) | 13.799 ± 0.168 | 0.044 ± 0.031 | 0.999 |
| Method | 5.0 g L−1–0.37 mAg:mLig | 10.0 g L−1–0.25 mAg:mLig | ||
|---|---|---|---|---|
| 8.4 mL | 84.0 mL | 8.4 mL | 84.0 mL | |
| UV-Vis (P+S) | 2.69 ± 0.07 | 2.76 ± 0.8 | 3.61 ± 0.16 | 3.77 ± 0.25 |
| UV-Vis (P) | 2.71 ± 0.05 | 2.67 ± 0.04 | 3.50 ± 0.05 | 3.45 ± 0.22 |
| DLS-Z-Average [nm] | 32.8 ± 3.4 | 36.2 ± 1.3 | 26.4 ± 1.6 | 27.4 ± 1.5 |
| DLS-PDI | 0.31 ± 0.05 | 0.40 ± 0.02 | 0.29 ± 0.05 | 0.32 ± 0.06 |
| Zeta potential [mV] | −31.2 ± 5.6 | −55.8 ± 2.1 | −41.1 ± 1.9 | −58.4 ± 1.9 |
| TEM core median size [nm] | 15 ± 3 | 13 ± 2 | 15 ± 2 | 12 ± 2 |
| TEM core median circularity | 0.991 ± 0.010 | 0.993 ± 0.016 | 0.990 ± 0.015 | 0.985 ± 0.018 |
| AAS-Lig-AgNPs yield [%] | 96.1 ± 4.0 | 96.7 ± 4.0 | 99.5 ± 4.0 | 98.9 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maršík, D.; Thoresen, P.P.; Maťátková, O.; Masák, J.; Sialini, P.; Rova, U.; Tsikourkitoudi, V.; Christakopoulos, P.; Matsakas, L.; Jarošová Kolouchová, I. Synthesis and Characterization of Lignin-Silver Nanoparticles. Molecules 2024, 29, 2360. https://doi.org/10.3390/molecules29102360
Maršík D, Thoresen PP, Maťátková O, Masák J, Sialini P, Rova U, Tsikourkitoudi V, Christakopoulos P, Matsakas L, Jarošová Kolouchová I. Synthesis and Characterization of Lignin-Silver Nanoparticles. Molecules. 2024; 29(10):2360. https://doi.org/10.3390/molecules29102360
Chicago/Turabian StyleMaršík, Dominik, Petter Paulsen Thoresen, Olga Maťátková, Jan Masák, Pavel Sialini, Ulrika Rova, Vasiliki Tsikourkitoudi, Paul Christakopoulos, Leonidas Matsakas, and Irena Jarošová Kolouchová. 2024. "Synthesis and Characterization of Lignin-Silver Nanoparticles" Molecules 29, no. 10: 2360. https://doi.org/10.3390/molecules29102360





