Understanding Chromium Slag Recycling with Sintering–Ironmaking Processes: Influence of Cr2O3 on the Sinter Microstructure and Mechanical Properties of the Silico–Ferrite of Calcium and Aluminum (SFCA)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mineral Composition and Microstructure of the Sinter
2.1.1. Mineral Composition of the Sinter
2.1.2. Microstructure of Sinters
2.2. Mineral Composition of Sinter
2.3. Mineral Composition and Microstructure of the Sinter
2.3.1. Effect of Cr2O3 on Microhardness of SFCA
2.3.2. Effect of Cr2O3 on the Compressive Strength of Sinter
3. Experimental Section
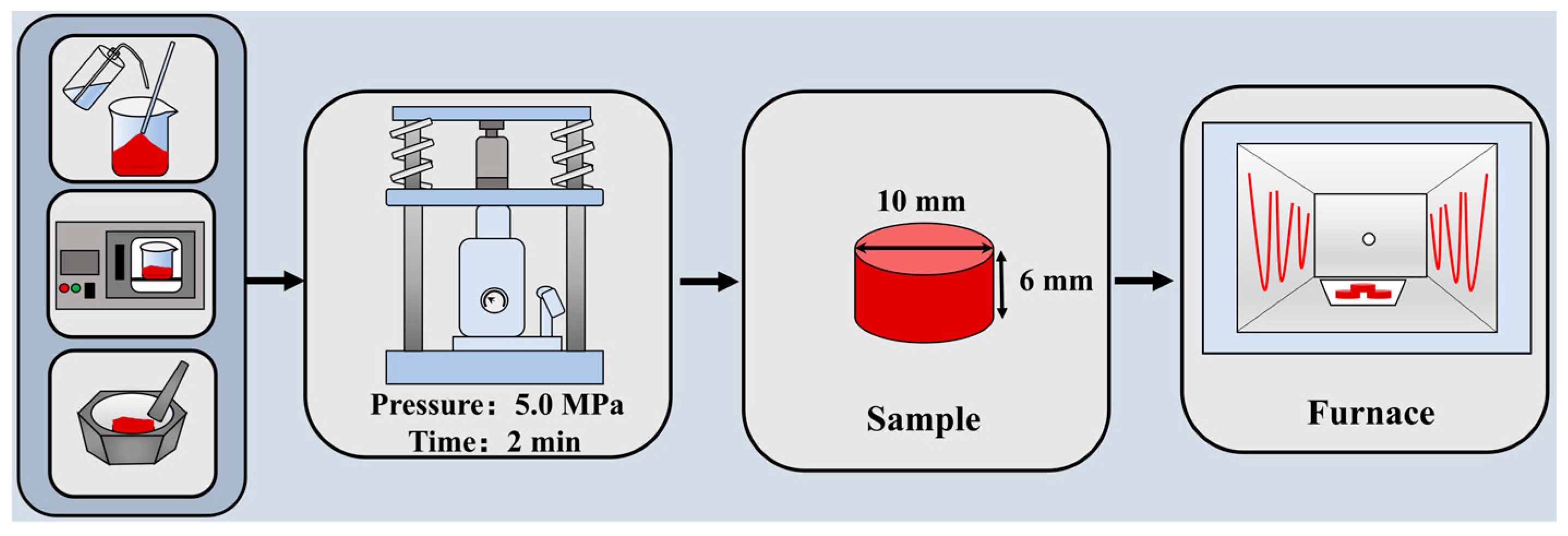
3.1. Sample Preparation
3.2. Characterization of Sintered Samples
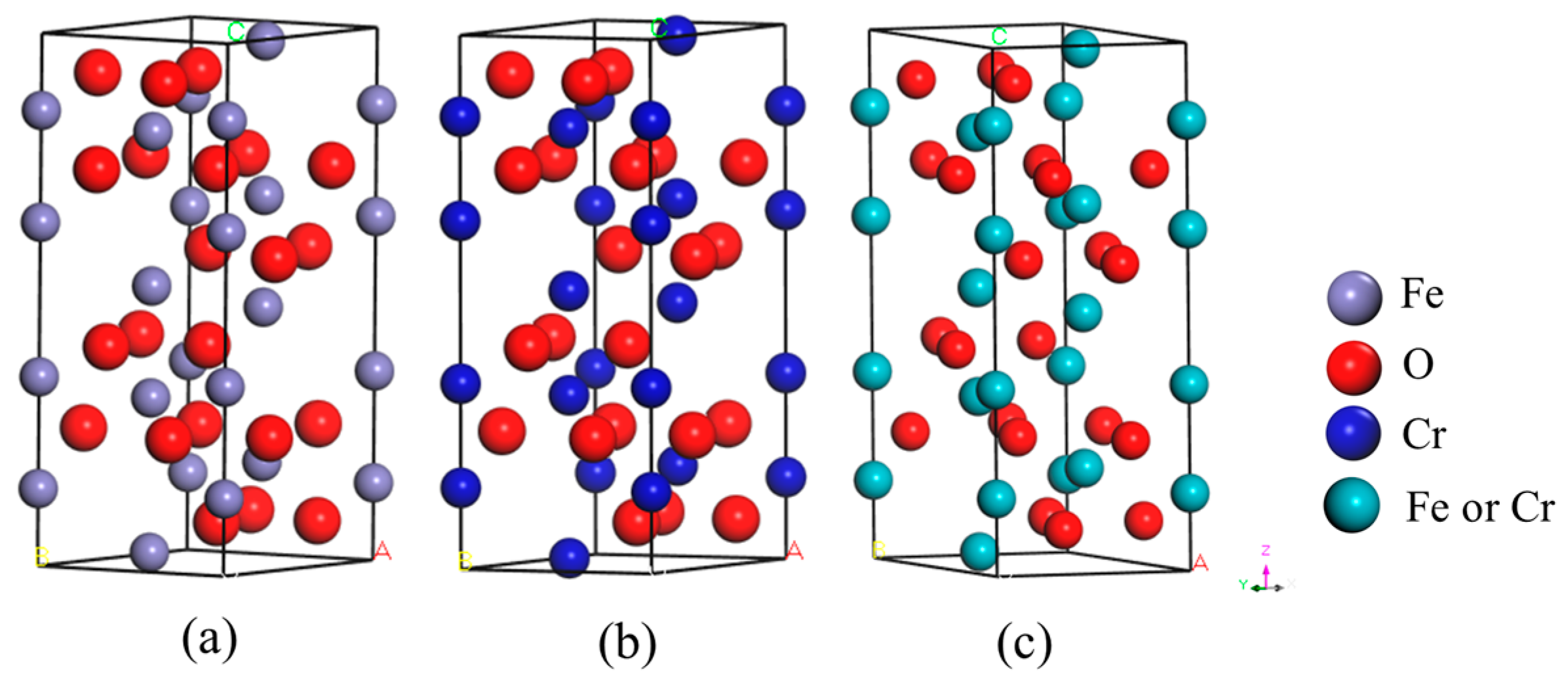
3.3. Method and Model of First-Principles Calculation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Z.W.; Wang, L.C.; Gu, F.Q.; Tang, H.M.; Rao, M.J.; Zhang, Y.B.; Li, G.H.; Jiang, T. Recovery of chromium from ferronickel slag: A comparison of microwave roasting and conventional roasting strategies. Powder Technol. 2020, 372, 578–584. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Zhang, L.L.; Cang, D.Q. Pilot trial of detoxification of chromium slag in cyclone furnace and production of slag wool fibres. J. Hazard. Mater. 2018, 358, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.; Huang, S.H.; Yang, Z.H.; Peng, B.; Huang, Y.; Chen, Y.H. Cr (VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J. Hazard. Mater. 2009, 167, 516–522. [Google Scholar] [CrossRef]
- Jagupilla, S.C.; Moon, D.H.; Wazne, M.; Christodoulatos, C.; Min, G.K. Effects of particle size and acid addition on the remediation of chromite ore processing residue using ferrous sulfate. J. Hazard. Mater. 2009, 168, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.N.; Li, C.L.; Yang, F. The disposition of chromite ore processing residue (COPR) incorporating industrial symbiosis. J. Clean. Prod. 2015, 95, 156–162. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, Q.; Yang, F.Y.; Bao, J.; Hu, Z.H.; Zhu, W.W.; Zhao, Y.Y.; Lin, Z.D.; Dong, Q.S. Chromium (VI) detoxification by oxidation and flocculation of exopolysaccharides from Arthrobacter sp. B4. J. Biol. Macromol. 2015, 81, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Hu, Y.J.; Zhang, X.; Sun, Y.Q.; Wu, Z.X.; Li, T.; Lv, J.T.; Li, J.L.; Zhang, J.; Zheng, L.R. Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ. Exp. Bot. 2018, 147, 43–52. [Google Scholar] [CrossRef]
- Wei, Y.F.; Wang, J.; Wang, J.X.; Zhan, L.; Ye, X.; Tan, H.B. Hydrothermal processing, characterization and leaching toxicity of Cr-added “fly ash-metakaolin” based geopolymer. Constr. Build. Mater. 2020, 251, 118931. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, Y.H.; Zhou, S.; Lei, X.R. Reduction/immobilization processes of hexavalent chromium using metakaolin-based geopolymer. J. Environ. Chem. Eng. 2016, 5, 373–380. [Google Scholar] [CrossRef]
- Tu, Y.K.; Su, Z.J.; Zhu, Y.X.; Zhang, Y.B.; Xu, J.; Jiang, T. A detoxification and value-added process for chromium ore processing residue (COPR) and Fe-C-bearing dust: Direct reduction-magnetic separation. Process. Saf. Environ. 2022, 167, 378–389. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.J.; Li, B.K.; Zevenhoven, R.; Saxén, H.; Jiang, M.F. Recovery of chromium from residue of sulfuric acid leaching of chromite. Process. Saf. Environ. 2018, 113, 78–87. [Google Scholar] [CrossRef]
- Luo, Z.T.; Zhi, T.Y.; Liu, L.; Mi, J.; Zhang, M.; Tian, C.F.; Si, Z.K.; Liu, X.H.; Mu, Y.D. Solidification/stabilization of chromium slag in red mud-based geopolymer. Constr. Build. Mater. 2022, 316, 125813. [Google Scholar] [CrossRef]
- Song, W.F.; Cao, J.W.; Wang, Z.; Geng, X.H.; Lu, J.S. Glass-ceramics microstructure formation mechanism for simultaneous solidification of chromium and nickel from disassembled waste battery and chromium slag. J. Hazard. Mater. 2021, 403, 123598. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.X.; Zhou, M.K.; Wang, H.D.; Liu, Z.H.; Wu, H.; Chen, X. Preparation and characterization of ceramic foams from chromium slag and coal bottom ash. Ceram. Int. 2018, 44, 11888–11891. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zhai, Y.B. Production practice of chromium slag sintered in Yusteel. Gansu. Metall. 2019, 41, 101–103. (In Chinese) [Google Scholar] [CrossRef]
- Chen, B.J.; Zhou, M.; Jiang, T.; Li, L. Observation of diffusion behavior between Cr2O3 and calcium ferrite based on diffusion couple method at 1373 K. J. Alloys Compd. 2019, 802, 103–111. [Google Scholar] [CrossRef]
- Chen, B.J.; Jiang, T.; Zhou, M.; Li, L.; Wen, J.; Wen, Y.C. Interdiffusion kinetics and solid-state reaction mechanism between Cr2O3 and calcium ferrite based on diffusion couple method. J. Alloys Compd. 2021, 865, 158754. [Google Scholar] [CrossRef]
- Dang, J. The experiment study on the chromium slag sintering pot. Jiugang. Technol. 2020, 5, 14–18. (In Chinese) [Google Scholar]
- Park, J.; Kim, E.; Suh, I.K.; Lee, J. Effects of basicity and Al2O3 content on the crystal structure of silico-ferrite of calcium and aluminum. ISIJ Int. 2023, 63, 235–243. [Google Scholar] [CrossRef]
- Yajima, K.; Jung, S.M. Data arrangement and consideration of evaluation standard for silico-ferrite of calcium and aluminum (SFCA) phase in sintering process. ISIJ Int. 2012, 52, 535–537. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Zhao, Y.J.; Gan, M.; Fan, X.H.; Chen, X.L.; Hu, L. Microstructure and minerals evolution of iron ore sinter: Influence of SiO2 and Al2O3. Minerals 2019, 9, 449. [Google Scholar] [CrossRef]
- Higuchi, K.; Naito, M.; Nakano, M.; Takamoto, Y. Optimization of chemical composition and microstructure of iron ore sinter for low-temperature drip of molten iron with high permeability. ISIJ Int. 2004, 44, 2057–2066. [Google Scholar] [CrossRef]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Liu, X.Q.; Li, S.W. Combined effect of MgO and basicity varied by different dolomite and burnt lime addition on sintering performance of magnetite concentrates. Ironmak. Steelmak. 2019, 47, 567–573. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.T.; Jiang, T.; Xue, X.X. Influence of MgO in form of magnesite on properties and mineralogy of high chromium, vanadium, titanium magnetite sinters. Ironmak. Steelmak. 2015, 42, 320. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.W.; Ou-Yang, Z.L.; Xu, R.S.; Song, M.M. Effect of SiO2 on the formation of acicular calcium ferrite in sinter. Metall. Mater. Trans. B 2019, 50, 678–687. [Google Scholar] [CrossRef]
- Yang, D.W.; Wang, W.; Xu, R.S.; Li, J.X.; Song, M.M. Effect of SiO2 on the mechanical property and reduction of calcium ferrite. Metals 2019, 9, 152. [Google Scholar] [CrossRef]
- Tang, W.D.; Xue, X.X.; Yang, S.T.; Zhang, L.H.; Huang, Z. Influence of basicity and temperature on bonding phase strength, microstructure, and mineralogy of high-chromium vanadium-titanium magnetite. Int. J. Min. Met. Mater. 2018, 25, 871–880. [Google Scholar] [CrossRef]
- Zhang, L.H.; Gao, Z.X.; Yang, S.T.; Tang, W.D.; Xue, X.X. Effect of basicity on sintering behavior and metallurgical properties of high-chromium vanadium-titanium magnetite. Metals 2020, 10, 569. [Google Scholar] [CrossRef]
- Tu, Y.K.; Su, Z.J.; Zhang, Y.B.; Jiang, T. Detoxication and recycling of chromium slag and C-bearing dust via composite agglomeration process (CAP)-blast furnace method. Waste Manag. 2023, 171, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Liu, T.T.; Pan, F.S. Research progress on intermetallic compounds and solid solutions of Mg alloys based on first-principles calculation. J. Chongqing Univ. 2018, 41, 30–44. (In Chinese) [Google Scholar]
- Bai, J.; Wu, M.; He, Q.; Wang, H.; Liao, Y.; Chen, L.; Chen, S. Emerging doped metal-organic frameworks: Recent progress in synthesis, applications, and first-principles calculations. Small 2024, e2306616. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.H.; Jia, L.; Lu, Z.L.; Chen, B.; Kondoh, K.; Cui, J. First-principles study and its experimental verification on the strength and ductility of O/Si solid solution strengthened Ti alloys. J. Mater. Res. Technol. 2023, 27, 7778–7786. [Google Scholar] [CrossRef]
- Roy, I.; Ekuma, C.; Balasubramanian, G. Examining the thermodynamic stability of mixed principal element oxides in AlCoCrFeNi high-entropy alloy by first-principles. Comput. Mater. Sci. 2022, 213, 111619. [Google Scholar] [CrossRef]
- Yang, D.W.; Wang, W.; Li, J.X.; Xu, R.H.; Wang, X.H.; Wang, G. Effect and mechanism of alumina on the morphology and mechanical properties of calcium ferrite. Metall. Mater. Trans. B 2020, 51, 776–785. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Xu, J.; Xu, J. Effect of Fe/Cr molar ratio and calcination temperature on preparation of black ceramic pigment with stainless steel dust assisted by microwave processing. J. Clean. Prod. 2022, 372, 133751. [Google Scholar] [CrossRef]
- Tsokov, P.; Blaskov, V.; Klissurski, D.; Tsolovski, I. Effect of mechanical activation on the synthesis of α-Fe2O3-Cr2O3 solid solutions. J. Mater. Sci. 1993, 28, 184–188. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, S.L.; Wang, Y.; Saengdeejing, A.; Chen, L.Q.; Liu, Z.K. First-principles calculations of the elastic, phonon and thermodynamic properties of Al12Mg12. Acta Mater. 2010, 58, 4012–4018. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. The Dynamical Theory of Crystal Lattices; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Mouhat, F.; Coudert, F.X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Chen, K.Y.; Zhao, L.R. Elastic properties, thermal expansion coefficients and electronic structures of Ti0.75X0.5C carbides. J. Phys. Chem. Solids. 2007, 68, 1805–1811. [Google Scholar] [CrossRef]
- Lou, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRP Press: New York, NY, USA, 2007. [Google Scholar]
- Hu, T.H.; Li, Z.H.; Zhang, Q.F. First principles and molecular dynamics simulations of effect of dopants on properties of high strength steel for hydrogen storage vessels. Acta Phys. Sin. 2024, 73, 067101. (In Chinese) [Google Scholar] [CrossRef]
- Ye, T.Z.; Wang, Z.T.; Wu, Y.W.; Zhang, J.; Chen, P.; Wang, M.J.; Tian, W.X.; Su, G.H.; Qiu, S.Z. Molecular dynamics simulation of tensile deformation behavior of single-crystal Fe-Cr-Al before and after irradiation. J. Mater. Res. 2022, 38, 828–840. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.G.; Zhong, J.; Wang, L.; Wang, Y.P.; Shu, Z.L. First-principles investigation of Cr-doped Fe2B: Structural, mechanical, electronic and magnetic properties. J. Magn. Magn. Mater. 2018, 456, 150–159. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Zhang, D.X.; Wang, J.; Dong, K.Z.; Hao, A.M. First principles investigation on the elastic and electronic properties of Mn, Co, Nb, Mo doped LiFePO4. Comput. Mater. Sci. 2018, 155, 410–415. [Google Scholar] [CrossRef]
- Tu, Y.K.; Zhang, Y.B.; Su, Z.J.; Jiang, T. Mineralization mechanism of limonitic laterite sinter under different fuel dosage: Effect of FeO. Powder Technol. 2022, 398, 117064. [Google Scholar] [CrossRef]
- Xue, Y.X.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Tian, H.Y.; Shi, Y.; Lu, S.H. Effect of alumina occurrence on sintering performance of iron ores and its action mechanism. J. Mater. Res. Technol. 2021, 12, 1157–1170. [Google Scholar] [CrossRef]
- Xu, J.; Ma, G.J.; Zheng, D.L.; Zhang, X.; Hai, Y.H. Influence of chromium slag on the microstructure and metallurgical performance of sinter. Sinter. Pelletiz. 2023, 48, 16–23. (In Chinese) [Google Scholar]
- Wu, H.; Huang, S.R.; Zhu, C.Y.; Zhu, H.G.; Xie, Z.H. Influence of Cr content on the microstructure and mechanical properties of CrxFeNiCu high entropy alloys. Prog. Nat. Sci. 2020, 30, 239–245. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, C.X.; Lin, S.H.; Liu, G.H.; Zhang, B.H.; Shi, H.H.; Dong, N.; Yang, N.X.; Zhang, F.C.; Guo, X.; et al. Calculation of mechanical properties, electronic structure and optical properties of CsPbX3 (X = F, Cl, Br, I). Molecules 2023, 28, 7643. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.T.; Zhang, X.Z.; Nagaumi, H.M.; Zhang, M.H.; Zhou, P.F.; Wang, R.; Zhang, B. Exploring the relationship between the structural characteristics and mechanical behavior of multicomponent Fe-containing phases: Experimental studies and first-principles calculations. Molecules 2023, 28, 7141. [Google Scholar] [CrossRef]
- Hamilton, J.D.G.; Hoskins, B.F.; Mumme, W.G.; Borbidge, W.E.; Montague, M.A. The crystal structure and crystal chemistry of Ca2.3Mg0.8Al1.5Si1.1Fe8.3O20 (SFCA): Solid solution limits and selected phase relationships of SFCA in the SiO2-Fe2O3-CaO(-Al2O3). Neues. Jahrb. Für. Mineral. Abhandlungen. 1989, 161, 1–26. [Google Scholar]










| Location | Point | Fe | O | Ca | Si | Al | Cr | Phase |
|---|---|---|---|---|---|---|---|---|
| 5b | 1 | 50.5 | 49.5 | - | - | - | - | Hematite |
| 2 | 31.0 | 54.0 | 8.4 | 3.1 | 3.5 | - | SFCA | |
| 3 | - | 58.6 | 20.8 | 20.5 | - | - | Silicate | |
| 5d | 4 | 53.1 | 45.9 | - | - | - | 1.0 | (Fe1−xCrx)2O3 |
| 5 | 38.5 | 44.8 | 9.7 | 3.8 | 2.6 | 0.6 | SFCA | |
| 3 | - | 56.1 | 22.6 | 21.6 | - | - | Silicate | |
| 5f | 4 | 51.0 | 47.5 | - | - | - | 1.6 | (Fe1−xCrx)2O3 |
| 5 | 42.9 | 49.7 | 4.4 | 1.6 | 2.0 | 0.9 | SFCA | |
| 3 | - | 58.5 | 27.0 | 13.7 | - | - | Silicate | |
| 5h | 4 | 49.3 | 48.5 | - | - | - | 2.2 | (Fe1−xCrx)2O3 |
| 5 | 35.5 | 50.7 | 8.6 | 2.5 | 1.0 | 1.4 | SFCA | |
| 3 | - | 56.0 | 29.3 | 14.7 | - | - | Silicate | |
| 5j | 4 | 49.7 | 47.1 | - | - | - | 3.2 | (Fe1−xCrx)2O3 |
| 5 | 41.1 | 46.6 | 5.0 | 2.7 | 2.5 | 2.7 | SFCA | |
| 3 | - | 67.6 | 21.0 | 11.4 | - | - | Silicate |
| Doped Cr Concentrations | a/Å | b/Å | c/Å | α/(°) | β/(°) | γ/(°) | V/Å3 |
|---|---|---|---|---|---|---|---|
| 0 at.% | 8.824 | 9.830 | 10.560 | 60.157 | 74.223 | 66.567 | 726.213 |
| 0.73 at.% | 17.656 * | 9.816 | 10.578 | 60.229 | 74.16 | 66.68 | 1454.73 * |
| 1.4 at.% | 8.819 | 9.809 | 10.591 | 60.317 | 74.215 | 66.678 | 727.921 |
| 2.94 at.% | 8.754 | 9.892 | 10.741 | 59.313 | 74.00 | 66.415 | 730.033 |
| 4.4 at.% | 8.764 | 9.859 | 10.732 | 59.755 | 73.788 | 66.764 | 732.551 |
| Cij | 0 at.% | 0.73 at.% | 1.4 at.% | 2.94 at.% | 4.4 at.% |
|---|---|---|---|---|---|
| C11 | 256.568 | 257.856 | 245.337 | 223.854 | 211.351 |
| C22 | 270.272 | 268.028 | 263.525 | 218.938 | 230.516 |
| C33 | 241.514 | 227.568 | 235.047 | 227.912 | 227.579 |
| C44 | 77.589 | 66.428 | 61.900 | 55.212 | 59.503 |
| C55 | 71.859 | 68.499 | 70.849 | 54.912 | 48.917 |
| C66 | 75.784 | 68.652 | 69.669 | 50.645 | 58.813 |
| C12 | 137.168 | 129.89 | 128.352 | 104.603 | 106.489 |
| C13 | 108.344 | 111.985 | 111.342 | 104.872 | 110.914 |
| C14 | 7.399 | 6.114 | 0.361 | 6.397 | 1.088 |
| C15 | −4.516 | −3.137 | −5.609 | −5.878 | −9.866 |
| C16 | −9.782 | −1.456 | −6.548 | −9.726 | −10.157 |
| C23 | 116.799 | 110.810 | 112.913 | 109.953 | 114.901 |
| C24 | −7.115 | −4.727 | −15.987 | 3.942 | −5.904 |
| C25 | 4.668 | 7.505 | 5.113 | −0.783 | −6.277 |
| C26 | −24.12 | 14.140 | −20.712 | 1.803 | −4.272 |
| C34 | −1.612 | −2.667 | −8.373 | 0.779 | −8.557 |
| C35 | −2.660 | −4.662 | −2.214 | −0.390 | −3.566 |
| C36 | −7.844 | −5.225 | −3.955 | −3.450 | −4.281 |
| C45 | −2.177 | −1.191 | −1.576 | 0.923 | 3.262 |
| C46 | 1.519 | 4.522 | 3.213 | 1.010 | −0.159 |
| C56 | 3.151 | 0.641 | −0.125 | −0.912 | 0.663 |
| Samples | Fe2O3 | Al2O3 | CaO | SiO2 | Cr2O3 |
|---|---|---|---|---|---|
| S1 | 81.38 | 2.13 | 10.57 | 5.94 | 0 |
| S2 | 80.57 | 2.11 | 10.49 | 5.83 | 1 |
| S3 | 79.76 | 2.09 | 10.38 | 5.77 | 2 |
| S4 | 78.97 | 2.07 | 10.26 | 5.70 | 3 |
| S5 | 78.18 | 2.05 | 9.50 | 5.28 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Ma, G.; Liu, M.; Zhang, X.; Zheng, D.; Du, T.; Luo, Y.; Zhang, W. Understanding Chromium Slag Recycling with Sintering–Ironmaking Processes: Influence of Cr2O3 on the Sinter Microstructure and Mechanical Properties of the Silico–Ferrite of Calcium and Aluminum (SFCA). Molecules 2024, 29, 2382. https://doi.org/10.3390/molecules29102382
Xu J, Ma G, Liu M, Zhang X, Zheng D, Du T, Luo Y, Zhang W. Understanding Chromium Slag Recycling with Sintering–Ironmaking Processes: Influence of Cr2O3 on the Sinter Microstructure and Mechanical Properties of the Silico–Ferrite of Calcium and Aluminum (SFCA). Molecules. 2024; 29(10):2382. https://doi.org/10.3390/molecules29102382
Chicago/Turabian StyleXu, Ju, Guojun Ma, Mengke Liu, Xiang Zhang, Dingli Zheng, Tianyu Du, Yanheng Luo, and Wei Zhang. 2024. "Understanding Chromium Slag Recycling with Sintering–Ironmaking Processes: Influence of Cr2O3 on the Sinter Microstructure and Mechanical Properties of the Silico–Ferrite of Calcium and Aluminum (SFCA)" Molecules 29, no. 10: 2382. https://doi.org/10.3390/molecules29102382






