Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections
Abstract
:1. Introduction
2. Phage Therapy Acts as a Revitalized Weapon to Eliminate MDR Bacteria and Biofilms
2.1. History of Phage Therapy
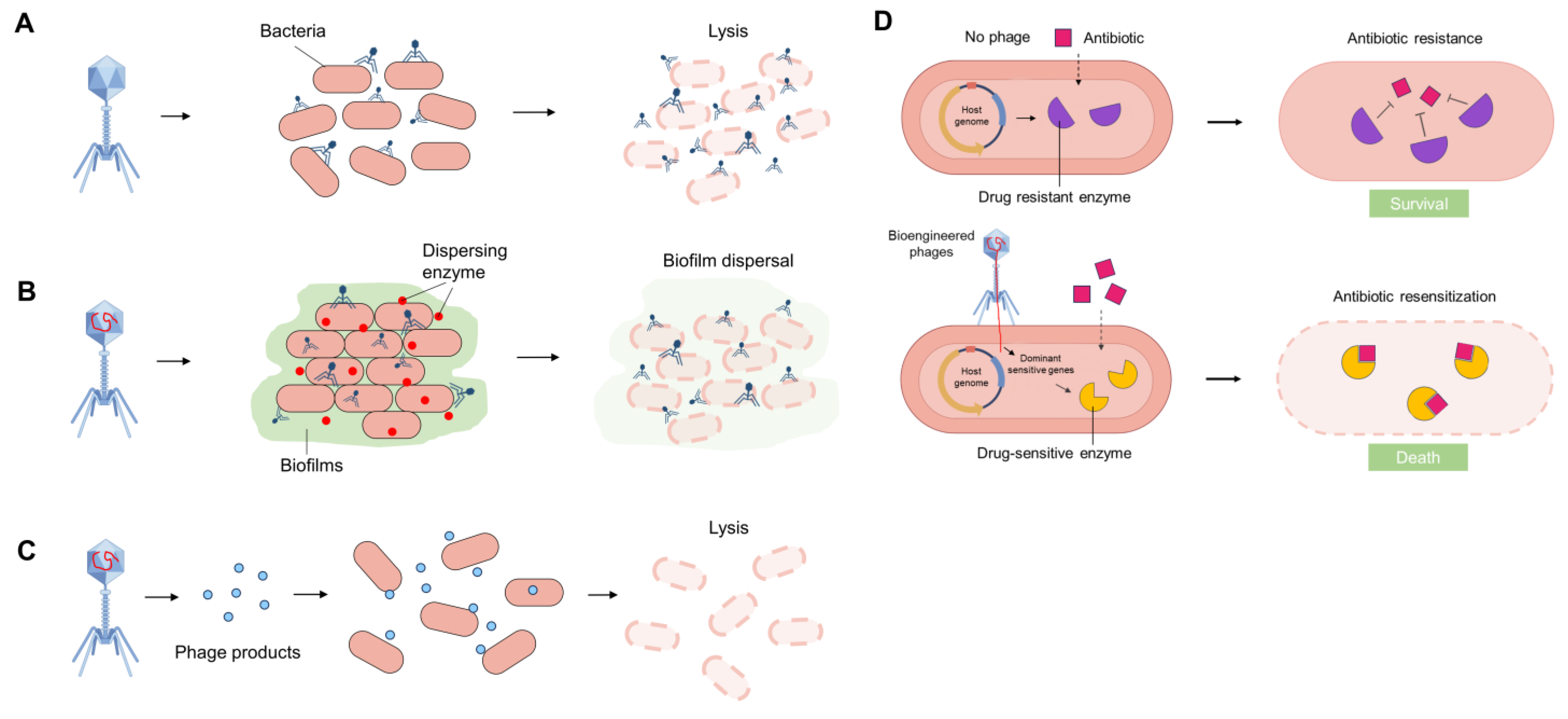
2.2. The Mechanisms and Characteristics of Phage Therapy
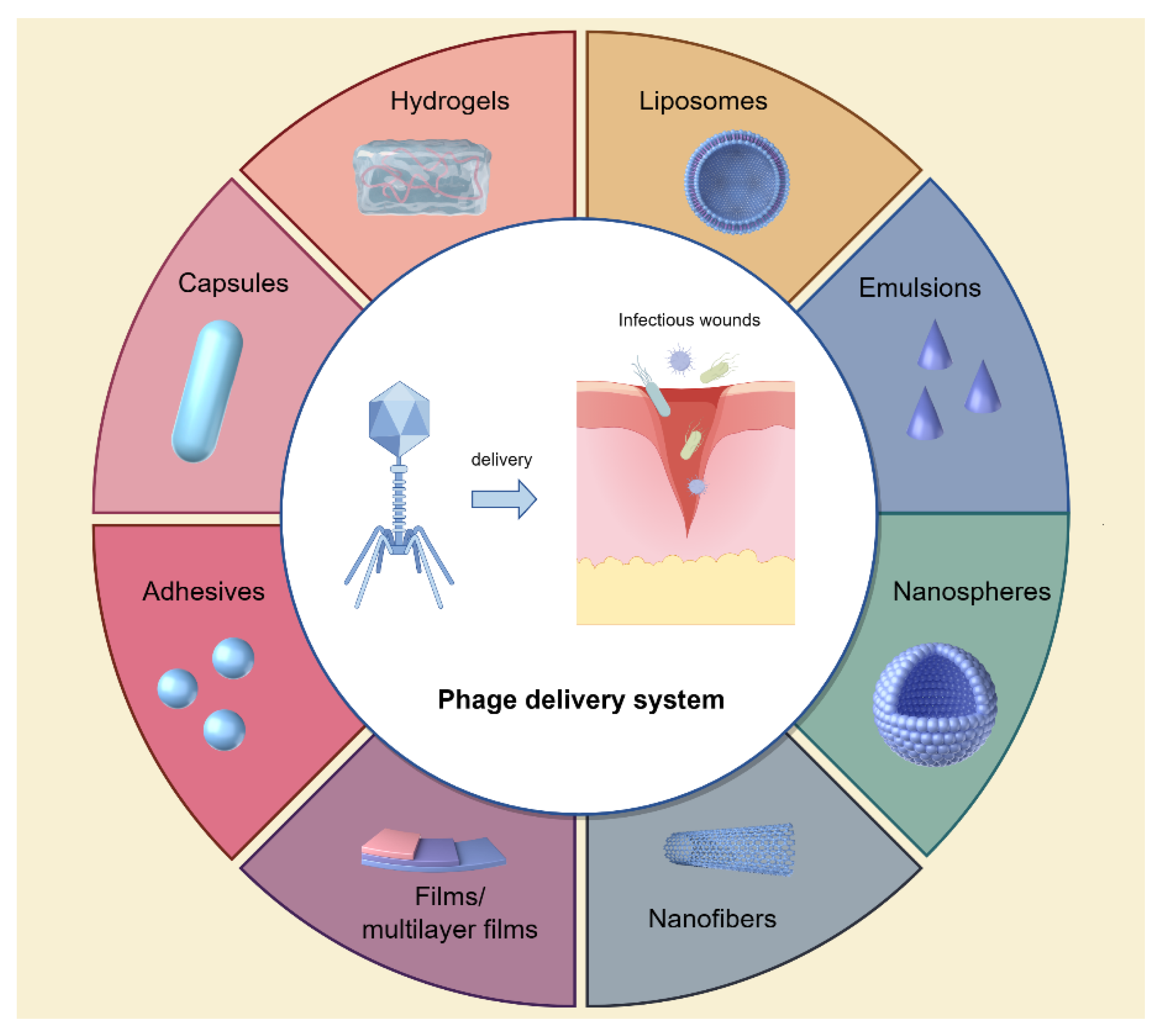
2.3. Phage Delivery Systems to Infectious Wounds
3. Clinical Application of Phage Therapy to Treat Refractory Wound Infections
3.1. Traumatic Wound Infections
3.2. Surgical Wound Infections
3.3. Burn Wound Infections
3.4. Diabetic Foot Infections
4. Perspectives and Challenges of Phage Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Brazil, J.C.; Quiros, M.; Nusrat, A.; Parkos, C.A. Innate immune cell-epithelial crosstalk during wound repair. J. Clin. Investig. 2019, 129, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound Dressing Modifications for Accelerated Healing of Infected Wounds. Int. J. Mol. Sci. 2023, 24, 7193. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef] [PubMed]
- George Broughton, I.I.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. S2), 5–15. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Michaels, J.A.; Campbell, W.B.; King, B.M.; Macintyre, J.; Palfreyman, S.J.; Shackley, P.; Stevenson, M.D. A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: The VULCAN trial. Health Technol. Assess. 2009, 13, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Møller, K.; Jensen, P.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Hill, K.E.; Malic, S.; Thomas, D.W.; Williams, D.W. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair. Regen. 2011, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Silva-Bermudez, P.; Jiménez-López, B.; Martínez-López, V.; Melgarejo-Ramírez, Y.; Brena-Molina, A.; Ibarra, C.; Baeza, I.; Martínez-Pardo, M.E.; Reyes-Frías, M.L.; et al. Silver-pig skin nanocomposites and mesenchymal stem cells: Suitable antibiofilm cellular dressings for wound healing. J. Nanobiotechnol. 2018, 16, 2. [Google Scholar] [CrossRef]
- Williams, M. Wound infections: An overview. Br. J. Community Nurs. 2021, 26, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Clinton, A.; Carter, T. Chronic Wound Biofilms: Pathogenesis and Potential Therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.J.; et al. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 November 2023).
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Oromí-Bosch, A.; Antani, J.D.; Turner, P.E. Developing Phage Therapy That Overcomes the Evolution of Bacterial Resistance. Annu. Rev. Virol. 2023, 10, 503–524. [Google Scholar] [CrossRef] [PubMed]
- D’Herelle, F. On an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. 1917. Res. Microbiol. 2007, 158, 553–554. [Google Scholar]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef]
- Khalid, A.; Lin, R.C.Y.; Iredell, J.R. A Phage Therapy Guide for Clinicians and Basic Scientists: Background and Highlighting Applications for Developing Countries. Front. Microbiol. 2020, 11, 599906. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Morais, A.P.; da Fonseca Batistão, D.W. Phage therapy as strategy to face post-antibiotic era: A guide to beginners and experts. Arch. Microbiol. 2021, 203, 1271–1279. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Letarov, A.V.; Kulikov, E.E. Adsorption of Bacteriophages on Bacterial Cells. Biochemistry 2017, 82, 1632–1658. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Qi, Y.; Yu, H.; Sun, W.; Raza, S.H.A.; Alkhorayef, N.; Alkhalil, S.S.; Salama, E.E.A.; Zhang, L. Bacteriophage Therapy as an Application for Bacterial Infection in China. Antibiotics 2023, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Friedman, N.; Molshanski-Mor, S.; Qimron, U. Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes. Appl. Env. Environ. Microbiol. 2012, 78, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Hauser, A.R.; Mecsas, J.; Moir, D.T. Beyond Antibiotics: New Therapeutic Approaches for Bacterial Infections. Clin. Infect. Dis. 2016, 63, 89–95. [Google Scholar] [CrossRef]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. A Systematic and Critical Review of Bacteriophage Therapy Against Multidrug-resistant ESKAPE Organisms in Humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Chang, B.J.; Payne, M.S. Applications for Bacteriophage Therapy during Pregnancy and the Perinatal Period. Front. Microbiol. 2017, 8, 2660. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Luong, T.; Darby, T.; Roach, D.R. Immunogenicity of bacteriophages. Trends Microbiol. 2023, 31, 1058–1071. [Google Scholar] [CrossRef]
- Hussain, W.; Yang, X.; Ullah, M.; Wang, H.; Aziz, A.; Xu, F.; Asif, M.; Ullah, M.W.; Wang, S. Genetic engineering of bacteriophages: Key concepts, strategies, and applications. Biotechnol. Adv. 2023, 64, 108116. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Topka, G.; Mantej, J.; Grabowski, Ł.; Necel, A.; Węgrzyn, G.; Węgrzyn, A. Propagation, Purification, and Characterization of Bacteriophages for Phage Therapy. Methods Mol. Biol. 2024, 2738, 357–400. [Google Scholar]
- Taati Moghadam, M.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Masjedian Jazi, F. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Brown, T.L.; Petrovski, S.; Chan, H.T.; Angove, M.J.; Tucci, J. Semi-Solid and Solid Dosage Forms for the Delivery of Phage Therapy to Epithelia. Pharm. 2018, 11, 26. [Google Scholar] [CrossRef]
- El-Shibiny, A.; El-Sahhar, S. Bacteriophages: The possible solution to treat infections caused by pathogenic bacteria. Can. J. Microbiol. 2017, 63, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Monserez, R.; van Belleghem, J.; Rose, T.; Jennes, S.; De Vos, D.; Verbeken, G.; Vaneechoutte, M.; Pirnay, J.P. Stability of bacteriophages in burn wound care products. PLoS ONE 2017, 12, e0182121. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Glasser, C.A.; Chaud, M.V.; del Fiol, F.S.; Tubino, M.; Vila, M.M. Biomimetic aqueous-core lipid nanoballoons integrating a multiple emulsion formulation: A suitable housing system for viable lytic bacteriophages. Colloids Surf. B Biointerfaces 2014, 123, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, Z.; Xu, R.; Cai, P.; Kristensen, P.; Chen, M.; Huang, Y. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional hydrogel-based wound dressings: A review on biocompatibility and therapeutic efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, R.Y.K.; Morales, S.; Chan, H.K. Bacteriophage-Delivering Hydrogels: Current Progress in Combating Antibiotic Resistant Bacterial Infection. Antibiotics 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; To, K.K.W.; Liu, Y.; Bai, C.; Leung, S.S.Y. A thermosensitive hydrogel formulation of phage and colistin combination for the management of multidrug-resistant Acinetobacter baumannii wound infections. Biomater. Sci. 2023, 12, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Banerjee, P.; Liu, Y.; Mi, Z.; Bai, C.; Hu, H.; To, K.K.W.; Duong, H.T.T.; Leung, S.S.Y. Development of thermosensitive hydrogel wound dressing containing Acinetobacter baumannii phage against wound infections. Int. J. Pharm. 2021, 602, 120508. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Kaur, J.; Kaur, S. Liposome Entrapment of Bacteriophages Improves Wound Healing in a Diabetic Mouse MRSA Infection. Front. Microbiol. 2018, 9, 561. [Google Scholar] [CrossRef]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef]
- Esteban, P.P.; Alves, D.R.; Enright, M.C.; Bean, J.E.; Gaudion, A.; Jenkins, A.T.; Young, A.E.; Arnot, T.C. Enhancement of the antimicrobial properties of bacteriophage-K via stabilization using oil-in-water nano-emulsions. Biotechnol. Prog. 2014, 30, 932–944. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef]
- Patel, J.C.; Mollitt, D.L.; Tepas, J.J., 3rd. Infectious complications in critically injured children. J. Pediatr. Surg. 2000, 35, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Krizek, T.J.; Robson, M.C. Evolution of quantitative bacteriology in wound management. Am. J. Surg. 1975, 130, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.R.; Jiang, F.; Badhiwala, J.H.; Schär, R.; Kurpad, S.; Grossman, R.G.; Harrop, J.S.; Guest, J.D.; Toups, E.G.; Shaffrey, C.I.; et al. Association of Pneumonia, Wound Infection, and Sepsis with Clinical Outcomes after Acute Traumatic Spinal Cord Injury. J. Neurotrauma 2019, 36, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J. Traumatic and surgical wounds. BMJ 2006, 332, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.F.; Rodeheaver, G.T.; Morgan, R.F.; Berman, D.E.; Thacker, J.G. Principles of emergency wound management. Ann. Emerg. Med. 1988, 17, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, S.K.; Prasad, R.; Sharma, S.; Shukla, V.; Nath, G.; Kumar, R. Biological Therapy on Infected Traumatic Wounds: A Case-Control Study. Int. J. Low. Extrem. Wounds 2022, 15347346211072779. [Google Scholar] [CrossRef] [PubMed]
- Racenis, K.; Rezevska, D.; Madelane, M.; Lavrinovics, E.; Djebara, S.; Petersons, A.; Kroica, J. Use of Phage Cocktail BFC 1.10 in Combination With Ceftazidime-Avibactam in the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Femur Osteomyelitis-A Case Report. Front Med. 2022, 9, 851310. [Google Scholar] [CrossRef]
- Chopra, H.; Islam, M.A.; Sharun, K.; Emran, T.B.; Al-Tawfiq, J.A.; Dhama, K. Recent advances in the treatment of biofilms induced surgical site infections. Int. J. Surg. 2023, 109, 65–67. [Google Scholar] [CrossRef]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Kochetkova, V.A.; Mamontov, A.S.; Moskovtseva, R.L.; Erastova, E.I.; Trofimov, E.I.; Popov, M.I.; Dzhubalieva, S.K. Phagotherapy of postoperative suppurative-inflammatory complications in patients with neoplasms. Sov. Med. 1989, 6, 23–26. [Google Scholar]
- Tsulukidze, A.P. Phage treatment in surgery. Surgery 1940, 12, 132–133. [Google Scholar]
- Ponomareva, T.R.; Smolianskaia, A.Z.; Sokolova, E.N.; Sokolova, V.I.; Garnova, N.A. Bacteriophages in the treatment of postoperative complications in cancer patients. Sov. Med. 1985, 4, 89–92. [Google Scholar]
- Moghadam, M.T.; Mojtahedi, A.; Salamy, S.; Shahbazi, R.; Satarzadeh, N.; Delavar, M.; Ashoobi, M.T. Phage therapy as a glimmer of hope in the fight against the recurrence or emergence of surgical site bacterial infections. Infection 2024, 52, e85661. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Nadareishvili, L.; Hoyle, N.; Nakaidze, N.; Nizharadze, D.; Kutateladze, M.; Balarjishvili, N.; Kutter, E.; Pruidze, N. Bacteriophage Therapy as a Potential Management Option for Surgical Wound Infections. Phage 2020, 1, 158–165. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Winkler, T.; Müller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, e14152. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, E.A.; Azzopardi, E.; Camilleri, L.; Villapalos, J.; Boyce, D.E.; Dziewulski, P.; Dickson, W.A.; Whitaker, I.S. Gram negative wound infection in hospitalised adult burn patients--systematic review and metanalysis. PLoS ONE 2014, 9, e95042. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.R.; Brennan, M.B. The role of the microbiome in nonhealing diabetic wounds. Ann. N. Y Acad. Sci. 2019, 1435, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Verbeken, G.; Vos, D.D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burn. Trauma. 2014, 4, 66–73. [Google Scholar]
- Rahimzadeh Torabi, L.; Doudi, M.; Naghavi, N.S.; Monajemi, R. Isolation, characterization, and effectiveness of bacteriophage PΦ-Bw-Ab against XDR Acinetobacter baumannii isolated from nosocomial burn wound infection. Iran. J. Basic. Med. Sci. 2021, 24, 1254–1263. [Google Scholar] [PubMed]
- McVay, C.S.; Velásquez, M.; Fralick, J.A. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob. Agents Chemother. 2007, 51, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Rezk, N.; Abdelsattar, A.S.; Elzoghby, D.; Agwa, M.M.; Abdelmoteleb, M.; Aly, R.G.; Fayez, M.S.; Essam, K.; Zaki, B.M.; El-Shibiny, A. Bacteriophage as a potential therapy to control antibiotic-resistant Pseudomonas aeruginosa infection through topical application onto a full-thickness wound in a rat model. J. Genet. Eng. Biotechnol. 2022, 20, 133. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Stacey, H.J.; Harkin, G.; Hall, L.M.L.; Young, M.J.; Jones, J.D. Patient perceptions of phage therapy for diabetic foot infection. PLoS ONE 2020, 15, e0243947. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Jneid, J.; Cassir, N.; Schuldiner, S.; Jourdan, N.; Sotto, A.; Lavigne, J.P.; La Scola, B. Exploring the Microbiota of Diabetic Foot Infections With Culturomics. Front. Cell Infect. Microbiol. 2018, 8, 282. [Google Scholar] [CrossRef]
- Fetni, S.; Ouahab, A.; Hamlaoui, F. Bacteriological profile and frequency of antibiotic resistance in the infected diabetic foot. Cell. Mol. Biol. 2023, 69, 143–149. [Google Scholar] [CrossRef]
- Mashaly, M.; Kheir, M.A.E.; Ibrahim, M.; Khafagy, W. Aerobic bacteria isolated from diabetic foot ulcers of Egyptian patients: Types, antibiotic susceptibility pattern and risk factors associated with multidrug-resistant organisms. Germs 2021, 11, 570–582. [Google Scholar] [CrossRef]
- Tascini, C.; Piaggesi, A.; Tagliaferri, E.; Iacopi, E.; Fondelli, S.; Tedeschi, A.; Rizzo, L.; Leonildi, A.; Menichetti, F. Microbiology at first visit of moderate-to-severe diabetic foot infection with antimicrobial activity and a survey of quinolone monotherapy. Diabetes Res. Clin. Pract. 2011, 94, 133–139. [Google Scholar] [CrossRef]
- Young, M.J.; Hall, L.M.L.; Merabishvilli, M.; Pirnay, J.P.; Clark, J.R.; Jones, J.D. Phage Therapy for Diabetic Foot Infection: A Case Series. Clin. Ther. 2023, 45, 797–801. [Google Scholar] [CrossRef]
- Mohamed, W.F.; Askora, A.A.; Mahdy, M.M.H.; El-Hussieny, E.A.; Abu-Shady, H.M. Isolation and Characterization of Bacteriophages Active against Pseudomonas aeruginosa Strains Isolated from Diabetic Foot Infections. Arch. Razi Inst. 2022, 77, 2187–2200. [Google Scholar]
- Gupta, P.; Singh, H.S.; Shukla, V.K.; Nath, G.; Bhartiya, S.K. Bacteriophage Therapy of Chronic Nonhealing Wound: Clinical Study. Int. J. Low. Extrem. Wounds 2019, 18, 171–175. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Rahimzadeh Torabi, L.; Doudi, M.; Naghavi, N.S.; Monajemi, R. Bacteriophages PΦEn-CL and PΦEn-HO can eliminate MDR Enterobacter cloacae and Enterobacter hormaechei isolated from burn wound infections without toxicity for human skin cells. FEMS Microbiol. Lett. 2021, 368, 212–228. [Google Scholar] [CrossRef]
- Prokopczuk, F.I.; Im, H.; Campos-Gomez, J.; Orihuela, C.J.; Martínez, E. Engineered Superinfective Pf Phage Prevents Dissemination of Pseudomonas aeruginosa in a Mouse Burn Model. mBio 2023, 14, e0047223. [Google Scholar] [CrossRef]
- Jokar, J.; Saleh, R.O.; Rahimian, N.; Ghasemian, A.; Ghaznavi, G.; Radfar, A.; Zarenezhad, E.; Najafipour, S. Antibacterial effects of single phage and phage cocktail against multidrug-resistant Klebsiella pneumoniae isolated from diabetic foot ulcer. Virus Genes. 2023, 59, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Kifelew, L.G.; Warner, M.S.; Morales, S.; Vaughan, L.; Woodman, R.; Fitridge, R.; Mitchell, J.G.; Speck, P. Efficacy of phage cocktail AB-SA01 therapy in diabetic mouse wound infections caused by multidrug-resistant Staphylococcus aureus. BMC Microbiol. 2020, 20, 204. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, 123–138. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e155226. [Google Scholar] [CrossRef]
- Malone, M.; Schultz, G. Challenges in the diagnosis and management of wound infection. Br. J. Dermatol. 2022, 187, 159–166. [Google Scholar] [CrossRef]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Smith, W.P.J.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef]
- Pires, D.P.; Dötsch, A.; Anderson, E.M.; Hao, Y.; Khursigara, C.M.; Lam, J.S.; Sillankorva, S.; Azeredo, J. A Genotypic Analysis of Five P. aeruginosa Strains after Biofilm Infection by Phages Targeting Different Cell Surface Receptors. Front. Microbiol. 2017, 8, 1229. [Google Scholar] [CrossRef]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhuyse, B.; Van der Linden, D.; Chatzis, O.; Lood, C.; Wagemans, J.; Lavigne, R.; Schroven, K.; Paeshuyse, J.; de Magnée, C.; Sokal, E.; et al. Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat. Commun. 2022, 13, 5725. [Google Scholar] [CrossRef] [PubMed]
- Akturk, E.; Melo, L.D.R.; Oliveira, H.; Crabbé, A.; Coenye, T.; Azeredo, J. Combining phages and antibiotic to enhance antibiofilm efficacy against an in vitro dual species wound biofilm. Biofilm 2023, 6, 100147. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Lin, Y.W.; Petrovic Fabijan, A.; Chang, R.Y.K.; Rao, G.G.; Iredell, J.; Chan, H.K.; Li, J. Pharmacokinetics/pharmacodynamics of phage therapy: A major hurdle to clinical translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef] [PubMed]


| Type | Isolated Bacteria | Phage Name | Main Results | Author Name | Reference |
|---|---|---|---|---|---|
| Traumatic wound infection | E. coli, P. aeruginosa K. pneumoniae, E. faecalis, and S. aureus | Non-specific phage cocktail | The wounds became sterile within 14 days of the customized BT and 81.5% of the wounds on BT could heal by primary intention. | Bhartiya et al. | [69] |
| MDR P. aeruginosa and carbapenem-resistant A. baumannii | Cocktail BFC 1.10 | Nine months after the intervention, the patient showed no signs of inflammation based on clinical, radiological, and laboratory assessments. | Karlis et al. | [70] | |
| Drug-resistant P. aeruginosa, S. aureus, and E. coli | - | A significant improvement was observed in the wound healing, and there were no signs of infection clinically and microbiologically after 3 to 5 doses of topical phage therapy. | Gupta et al. | [98] | |
| Surgical wound infection | Streptococcus spp., P. mirabilis, Enterococcus spp., E. coli, P. aeruginosa, and Staphylococcus spp. | Pyophage | 60% of patients exhibited a lack of urinary tract infection symptoms post treatment, along with a decrease in colony-forming units, as observed in microbiological outcomes. | Leitner et al. | [77] |
| MDR bacterial infection such as B. cepacia, S. aureus, and E. faecalis | Pyophage and antistaphylococcal phage | Phage therapy demonstrated a beneficial impact on all four cases, leading to an enhancement in overall health status and wound healing. | Nadareishyili et al. | [78] | |
| MDR P. aeruginosa | - | No bacterial isolates were detected in drainage fluids collected on days 3, 4, and 5 following phage treatment. | Tkhilaishvili et al. | [79] | |
| P. aeruginosa | Cocktail of 12 natural lytic phages | The phage therapy was effective on bacterial colonies isolated from wounds. | Jault et al. | [99] | |
| Burn wound infection | XDR A. baumannii strain | Phage PΦ-Bw-Ab | XDR baumannii strain IAU_FAL101 showed significant sensitivity to phage PΦ-Bw-Ab. | Doudi et al. | [87] |
| P. aeruginosa | P. aeruginosa phage cocktail | Administration of a single dose of the P. aeruginosa phage cocktail significantly reduced mortality in burn-injured mice infected with P. aeruginosa, with the intraperitoneal route providing the most effective protection. | Fralick et al. | [88] | |
| MDR Enterobacter cloacae and E. hormaechei | Phages PΦEn-CL and PΦEn-HO | E. cloacae strain Iau-EC100 and E. hormaechei strain Iau-EHO100 isolated from burn wounds were sensitive to the isolated phages. Phages had no significant toxicity effect on human skin cells | Torabi et al. | [100] | |
| P. aeruginosa PAO1 strain | Engineered superinfective Pf phage | Pf phage treatment completely abolished the capability of P. aeruginosa to disseminate from the burn site to internal organs. And over the course of 10 days, this resulted in bacterial clearance and survival of all treated mice. | Prokopczuk et al. | [101] | |
| Diabetic foot infection | S. aureus and S. hominis | Anti-staphylococcal phage | Most of the bacteria isolates of DFIs were susceptible to the phage, and the DFIs almost achieved clinical resolution with phage therapy. | Jones et al. | [96] |
| MDR P. aeruginosa | Phage ϕPAE1 and ϕPAE2 | Phages ϕPAE1 and ϕPAE2 exhibited broad antibacterial effects against P. aeruginosa clinical strains isolated from DFIs. | Mohamed et al. | [97] | |
| MDR K. pneumonia | Phage cocktail (KP1, KP2, KP3, and KP4) | Phage cocktail demonstrated significantly higher antibacterial activity than each single phage without any bacterial regrowth. | Jokar et al. | [102] | |
| XDR S. aureus | S. aureus myoviridae phages cocktail AB-SA01, | AB-SA01 treatment decreased the bacterial load with efficacy superior to vancomycin treatment, and no mortality was recorded to be associated with infections. | Kifelew et al. | [103] |
| Advantages | Current Problems |
|---|---|
| Clinical safety: relatively free of side effects; Specificity: does not kill microbial community; Low cost: easy and rapid isolation with lower costs; High efficacy: rapidly distribute throughout the body with an increased concentration at the site of infection; Potential reversion of bacterial antibiotic susceptibility; Biofilm degrading activity; Amenability to engineering. | High specificity: causative bacterium must be identified beforehand, narrow spectrum of action; Emergence of bacterial resistance during phage treatment; Not enough well-designed clinical trials supporting its efficacy and safety in clinical practice; Lack of in vivo PK/PD data and specific regulatory framework. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Du, L.; Dong, B.; Kou, E.; Wang, L.; Zhu, Y. Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections. Int. J. Mol. Sci. 2024, 25, 5465. https://doi.org/10.3390/ijms25105465
Wang B, Du L, Dong B, Kou E, Wang L, Zhu Y. Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections. International Journal of Molecular Sciences. 2024; 25(10):5465. https://doi.org/10.3390/ijms25105465
Chicago/Turabian StyleWang, Bo, Lin Du, Baiping Dong, Erwen Kou, Liangzhe Wang, and Yuanjie Zhu. 2024. "Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections" International Journal of Molecular Sciences 25, no. 10: 5465. https://doi.org/10.3390/ijms25105465




