Diatom Biosilica Functionalised with Metabolically Deposited Cerium Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Cultivation of Diatom Species Pseudostaurosira trainorii
2.2. Procedure for Cleaning Diatom Frustules
2.3. Methods of the Material Characterisation
3. Results and Discussion
3.1. Kinetics of Cerium Sorption by Diatom Cells from the Culture Medium and Dependence on the Harvested Diatom Biomass on the Concentration Ratio Si/Ce in Culture Medium
3.2. SEM and SEM-EDX Studies of Synthesised Composites
3.3. Transmission Electron Microscopy Study
3.4. Powder X-ray Diffraction Analysis
3.5. Thermogravimetric Analysis
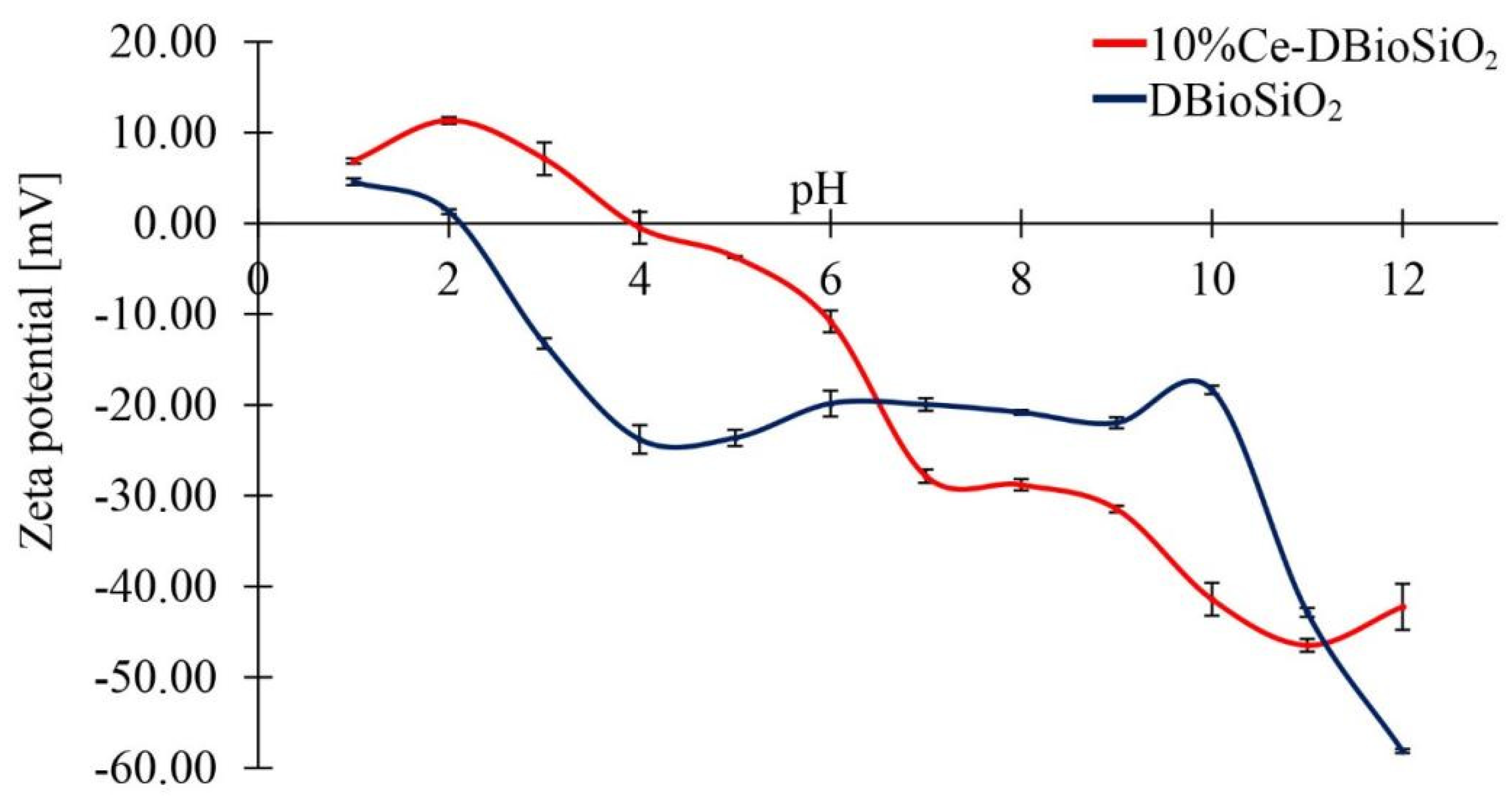
3.6. Zeta Potential Measurements
3.7. FTIR Spectra
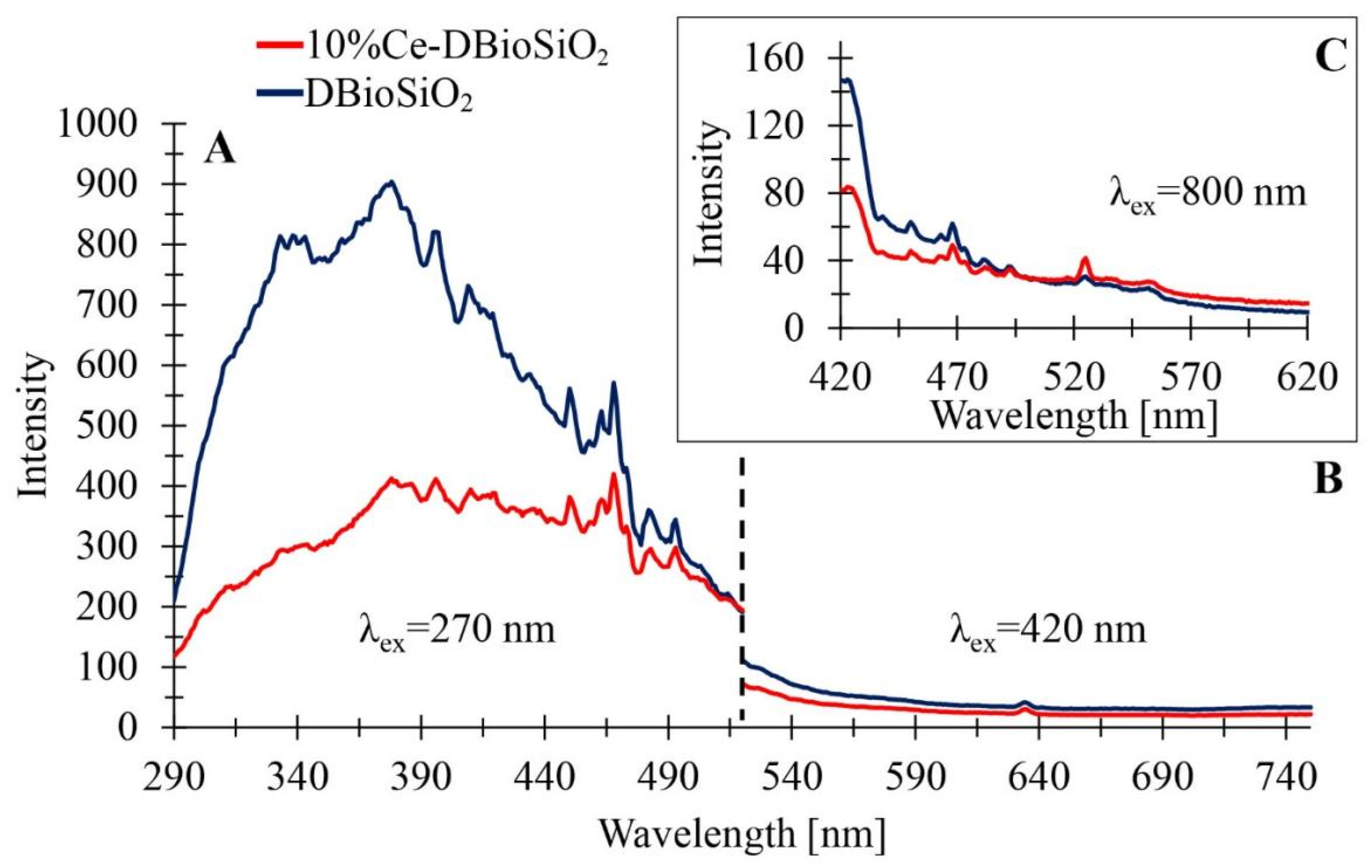
3.8. Photoluminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green synthesis of cerium oxide nanoparticles (Ceo2 nps) and their antimicrobial applications: A review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Boaro, M.; Colussi, S.; de Leitenburg, C.; Trovarelli, A. Ceria-Based Materials in Catalysis: Historical Perspective and Future Trends. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 209–242. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M. Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J. Photochem. Photobiol. B 2016, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Hernández, A.; Annangi, B.; Demir, E.; Bach, J.; Rubio, L.; Creus, A.; Marcos, R. Antioxidant and antigenotoxic properties of CeO2 NPs and cerium sulphate: Studies with Drosophila melanogaster as a promising in vivo model. Nanotoxicology 2015, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Saikia, H.; Hazarika, K.K.; Chutia, B.; Choudhury, B.; Bharali, P. A Simple Chemical Route toward High Surface Area CeO2 Nanoparticles Displaying Remarkable Radical Scavenging Activity. ChemistrySelect 2017, 2, 3369–3375. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimeŕs Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Hijaz, M.; Das, S.; Mert, I.; Gupta, A.; Al-Wahab, Z.; Tebbe, C.; Dar, S.; Chhina, J.; Giri, S.; Munkarah, A.; et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Zhou, M.; Huang, Z.; Gao, J.; Ma, Z.; Chen, J.; Tang, X. Enhanced Performance of Ceria-Based NOx Reduction Catalysts by Optimal Support Effect. Environ. Sci. Technol. 2017, 51, 473–478. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhang, D.; Lan, Y.; Guo, J. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate. J. Hazard. Mater. 2020, 381, 121209. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.D.; Yan, C.H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Zhang, G.; Ao, J.; Guo, Y.; Zhang, Z.; Shao, M.; Wang, L.; Zhou, L.; Shao, J. Green synthesis and catalytic performance of nanoscale CeO2 sheets. RSC Adv. 2014, 4, 20131–20135. [Google Scholar] [CrossRef]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dabek, P.; Witkowski, A.; Buszewski, B. “Outsourcing” Diatoms in Fabrication of Metal-Doped 3D Biosilica. Materials 2020, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dąbek, P.; Markuszewski, M.J.; Witkowski, A.; Buszewski, B. Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials 2022, 15, 5210. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products. Appl. Microbiol. Biotechnol. 2003, 60, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Marella, T.K.; Tao, L.; Li, R.; Tiwari, A.; Li, G. Optimization of growth conditions and fatty acid analysis for three freshwater diatom isolates. Phycol. Res. 2017, 65, 177–187. [Google Scholar] [CrossRef]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales. Appl. Microbiol. Biotechnol. 2003, 60, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Thamatrakoln, K.; Hildebrand, M. Silicon Uptake in Diatoms Revisited: A Model for Saturable and Nonsaturable Uptake Kinetics and the Role of Silicon Transporters. Plant Physiol. 2008, 146, 1397–1407. [Google Scholar] [CrossRef]
- Ketheesan, B.; Nirmalakhandan, N. Modeling microalgal growth in an airlift-driven raceway reactor. Bioresour. Technol. 2013, 136, 689–696. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 1–719. [Google Scholar] [CrossRef]
- Morales, E.A. Morphological studies in selected fragilarioid diatoms (Bacillariophyceae) from Connecticut waters (U.S.A.). Proc. Acad. Nat. Sci. Phila. 2001, 151, 105–120. [Google Scholar] [CrossRef]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Mouchet, F.; Verneuil, L.; Evariste, L.; Silvestre, J.; Pinelli, E.; Gauthier, L. Toxicity of CeO2 nanoparticles at different trophic levels—Effects on diatoms, chironomids and amphibians. Chemosphere 2015, 120, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Z.; Liu, C.; Wang, F.; Zhang, Y.; Wang, M. Biotemplated fabrication of hierarchical mesoporous CeO2 derived from diatom and its application for catalytic oxidation of CO. Chin. Sci. Bull. 2014, 59, 3260–3265. [Google Scholar] [CrossRef]
- Marlow, W.H. Van der Waals Energies in the Formation and Interaction of Nanoparticle Aggregates. In Gas Phase Nanoparticle Synthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 1–27. [Google Scholar] [CrossRef]
- Wojtczak, I.; Brzozowska, W.; Railean, V.; Bekissanova, Z.; Trykowski, G.; Sprynskyy, M. Diatomaceous Biosilica Doped with Heteroepitaxially Growing Ag/AgCl/CeO2 Composite Nanoparticles: Synthesis, Characterisation and Antibacterial Application. J. Clust. Sci. 2024, 35, 429–442. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef]
- Ioannou, M.E.; Pouroutzidou, G.K.; Chatzimentor, I.; Tsamesidis, I.; Florini, N.; Tsiaoussis, I.; Lymperaki, E.; Komninou, P.; Kontonasaki, E. Synthesis and Characterization of Cerium Oxide Nanoparticles: Effect of Cerium Precursor to Gelatin Ratio. Appl. Sci. 2023, 13, 2676. [Google Scholar] [CrossRef]
- Sreeremya, T.S.; Krishnan, A.; Remani, K.C.; Patil, K.R.; Brougham, D.F.; Ghosh, S. Shape-selective oriented cerium oxide nanocrystals permit assessment of the effect of the exposed facets on catalytic activity and oxygen storage capacity. ACS Appl. Mater. Interfaces 2015, 7, 8545–8555. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal Ceria Nanocrystals: A Tailor-Made Crystal Morphology in Supercritical Water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Ansari, A.A.; Labis, J.; Alam, M.; Ramay, S.M.; Ahmad, N.; Mahmood, A. Effect of cobalt doping on structural, optical and redox properties cerium oxide nanoparticles. Phase Transit. 2016, 89, 261–272. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Liu, W.; Wang, S.; Xie, A.; Liu, X.; Wang, J.; Yang, Y. Facile Preparation of Mn+-Doped (M = Cu, Co, Ni, Mn) Hierarchically Mesoporous CeO2 Nanoparticles with Enhanced Catalytic Activity for CO Oxidation. Eur. J. Inorg. Chem. 2015, 2015, 969–976. [Google Scholar] [CrossRef]
- Brugnoli, L.; Urata, S.; Pedone, A. H2O2 adsorption and dissociation on various CeO2 surface models: A first-principles study. J. Phys. Condens. Matter 2022, 34, 164006. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Yu, K.; Hu, S.; Chen, F. Adsorption-depended Fenton-like reaction kinetics in CeO2-H2O2 system for salicylic acid degradation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 456–463. [Google Scholar] [CrossRef]
- Breznan, D.; Nazemof, N.; Kunc, F.; Hill, M.; Vladisavljevic, D.; Gomes, J.; Johnston, L.J.; Vincent, R.; Kumarathasan, P. Acellular oxidative potential assay for screening of amorphous silica nanoparticles. Analyst 2020, 145, 4867–4879. [Google Scholar] [CrossRef] [PubMed]
- Kunc, F.; Balhara, V.; Sun, Y.; Daroszewska, M.; Jakubek, Z.J.; Hill, M.; Brinkmann, A.; Johnston, L.J. Quantification of surface functional groups on silica nanoparticles: Comparison of thermogravimetric analysis and quantitative NMR. Analyst 2019, 144, 5589–5599. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.; Kumar, R.; Mehta, S.K. Nanoscale surface designing of Cerium oxide nanoparticles for controlling growth, stability, optical and thermal properties. Ceram. Int. 2015, 41, 10995–11003. [Google Scholar] [CrossRef]
- Bourja, L.; Bakiz, B.; Benlhachemi, A.; Ezahri, M.; Villain, S.; Favotto, C.; Valmalette, J.C.; Gavarri, J.R. Structural modifications of nanostructured ceria CeO2,xH2O during dehydration process. Powder Technol. 2012, 215–216, 66–71. [Google Scholar] [CrossRef]
- Phonthammachai, N.; Rumruangwong, M.; Gulari, E.; Jamieson, A.M.; Jitkarnka, S.; Wongkasemjit, S. Synthesis and rheological properties of mesoporous nanocrystalline CeO2 via sol–gel process. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 61–68. [Google Scholar] [CrossRef]
- Zdravković, J.; Simović, B.; Golubović, A.; Poleti, D.; Veljković, I.; Šćepanović, M.; Branković, G. Comparative study of CeO2 nanopowders obtained by the hydrothermal method from various precursors. Ceram. Int. 2015, 41, 1970–1979. [Google Scholar] [CrossRef]
- Kujawa, J.; Al-Gharabli, S.; Wrzeszcz, G.; Knozowska, K.; Lagzdins, R.; Talik, E.; Dziedzic, A.; Loulergue, P.; Szymczyk, A.; Kujawski, W. Physicochemical and magnetic properties of functionalized lanthanide oxides with enhanced hydrophobicity. Appl. Surf. Sci. 2021, 542, 148563. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Hossain, M.A.; Chong, S.L.; Soh, J.C.; Abdullah, S.; Khan, M.R.; Cheng, C.K. Non-isothermal kinetics and mechanistic study of thermal decomposition of light rare earth metal nitrate hydrates using thermogravimetric analysis. J. Therm. Anal. Calorim. 2016, 125, 423–435. [Google Scholar] [CrossRef]
- Lundy, R.; Byrne, C.; Bogan, J.; Nolan, K.; Collins, M.N.; Dalton, E.; Enright, R. Exploring the Role of Adsorption and Surface State on the Hydrophobicity of Rare Earth Oxides. ACS Appl. Mater. Interfaces 2017, 9, 13751–13760. [Google Scholar] [CrossRef]
- Khalil, K.M.S.; Elkabee, L.A.; Murphy, B. Formation and characterization of different ceria/silica composite materials via dispersion of ceria gel or soluble ceria precursors in silica sols. J. Colloid Interface Sci. 2005, 287, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.A.; Shin, M.; Juang, L.J.; Kastrup, C.J.; Go, G.M.; Lee, H. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. ACS Nano 2020, 14, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Dékány, I.; De Keizer, A. Adsorption of dodecyl pyridinium chloride on monodisperse porous silica. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 327–336. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Bekissanova, Z.; Railean, V.; Wojtczak, I.; Brzozowska, W.; Trykowski, G.; Ospanova, A.; Sprynskyy, M. Synthesis and Antimicrobial Activity of 3D Micro–Nanostructured Diatom Biosilica Coated by Epitaxially Growing Ag-AgCl Hybrid Nanoparticles. Biomimetics 2024, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kashyap, M.; Gautam, S.; Shukla, P.; Joshi, K.B.; Vinayak, V. Fast Fourier infrared spectroscopy to characterize the biochemical composition in diatoms. J. Biosci. 2018, 43, 717–729. [Google Scholar] [CrossRef]
- Zemnukhova, L.A.; Panasenko, A.E.; Tsoi, E.A.; Fedorishcheva, G.A.; Shapkin, N.P.; Artem’Yanov, A.P.; Maiorov, V.Y. Composition and structure of amorphous silica produced from rice husk and straw. Inorg. Mater. 2014, 50, 75–81. [Google Scholar] [CrossRef]
- Al Khafaji, S.J.S.; Ghobeh, M.; Mashergi, M.; Es-Haghi, A. Biological Synthesis of Cerium Oxide Nanoparticles Using Funnel Extract: Characterization and Evaluation of Its Angiogenesis and Cytotoxicity Properties Against Breast Cancer Cells. Bionanoscience 2024, 1–12. [Google Scholar] [CrossRef]
- De Stefano, L.; Rendina, I.; De Stefano, M.; Bismuto, A.; Maddalena, P. Marine diatoms as optical chemical sensors. Appl. Phys. Lett. 2005, 87, 233902. [Google Scholar] [CrossRef]
- Blahuta, S.; Bessière, A.; Viana, B.; Dorenbos, P.; Ouspenski, V. Evidence and consequences of Ce4+ in LYSO:Ce,Ca and LYSO:Ce,Mg single crystals for medical imaging applications. IEEE Trans. Nucl. Sci. 2013, 60, 3134–3141. [Google Scholar] [CrossRef]
- Wang, Z.; Quan, Z.; Lin, J. Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions doping. Inorg. Chem. 2007, 46, 5237–5242. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.C.; Moreno, O.P.; Mora-Ramírez, M.A.; Santiesteban, H.J.; Avendaño, C.B.; Bernal, Y.P. Optical and structural analysis of the charge transfer of Ce3++ e− →Ce4+ ion in the cerium oxide (CeO2). Optik 2021, 248, 168178. [Google Scholar] [CrossRef]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-controllable synthesis of mesoporous CeO2 nano- and microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Prasad, B.D.; Sharma, S.C.; Vidya, Y.S.; Anantharaju, K.S. Structural, photoluminescence and thermoluminescence properties of CeO2 nanoparticles. Optik 2016, 127, 855–861. [Google Scholar] [CrossRef]
- Anufrick, S.S.; Kurian, N.N.; Sergienko, I.G.; Anuchin, S.N. Morphology and Structure of Coatings Obtained by Laser Ablation of Rare-Earth Metals. J. Appl. Spectrosc. 2024, 90, 1229–1235. [Google Scholar] [CrossRef]
- Aitasalo, T.; Dereń, P.; Hölsä, J.; Jungner, H.; Krupa, J.C.; Lastusaari, M.; Legendziewicz, J.; Niittykoski, J.; Strȩk, W. Persistent luminescence phenomena in materials doped with rare earth ions. J. Solid State Chem. 2003, 171, 114–122. [Google Scholar] [CrossRef]
- Qin, T.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Photoluminescence of silica nanostructures from bioreactor culture of marine diatom Nitzschia frustulum. J. Nanosci. Nanotechnol. 2008, 8, 2392–2398. [Google Scholar] [CrossRef]
- Pacchioni, G.; Skuja, L.; Griscom, D.L. (Eds.) Defects in SiO2 and Related Dielectrics: Science and Technology; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]








| Name | ||||||
|---|---|---|---|---|---|---|
| 10%Ce-DBioSiO2 | 0.01154 | 0.59 | 5266.824 | 276,481.801 | 1.859 | 1.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtczak, I.; Brzozowska, W.; Trykowski, G.; Sprynskyy, M. Diatom Biosilica Functionalised with Metabolically Deposited Cerium Oxide Nanoparticles. Materials 2024, 17, 2390. https://doi.org/10.3390/ma17102390
Wojtczak I, Brzozowska W, Trykowski G, Sprynskyy M. Diatom Biosilica Functionalised with Metabolically Deposited Cerium Oxide Nanoparticles. Materials. 2024; 17(10):2390. https://doi.org/10.3390/ma17102390
Chicago/Turabian StyleWojtczak, Izabela, Weronika Brzozowska, Grzegorz Trykowski, and Myroslav Sprynskyy. 2024. "Diatom Biosilica Functionalised with Metabolically Deposited Cerium Oxide Nanoparticles" Materials 17, no. 10: 2390. https://doi.org/10.3390/ma17102390







