Keratin/Copper Complex Electrospun Nanofibers for Antibacterial Treatments: Property Investigation and In Vitro Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Keratin/Copper Complex Electrospun Nanofibers
- (a)
- 5 wt/v% copper(II) nitrate trihydrate, Cu(NO3)2·3H2O (CAS 10031-43-3, Sigma-Aldrich, Merck Life Science S.r.l., Milano, Italy);
- (b)
- 1 wt/v% copper (II) acetate Cu(CH3COO)2 (CAS 142-71-2, Sigma-Aldrich, Merck Life Science S.r.l., Milano, Italy);
- (c)
- 5 wt/v% copper(II) chloride, CuCl2 (CAS 7447-39-4, Sigma-Aldrich, Merck Life Science S.r.l., Milano, Italy).
2.2. Material Characterization
2.3. Antibacterial Tests
2.4. Cell Culture
2.5. Cytocompatibility Assays
3. Results and Discussion
3.1. Preliminary Morphological Screening of Different Treatments’ Influence on Keratin NFs
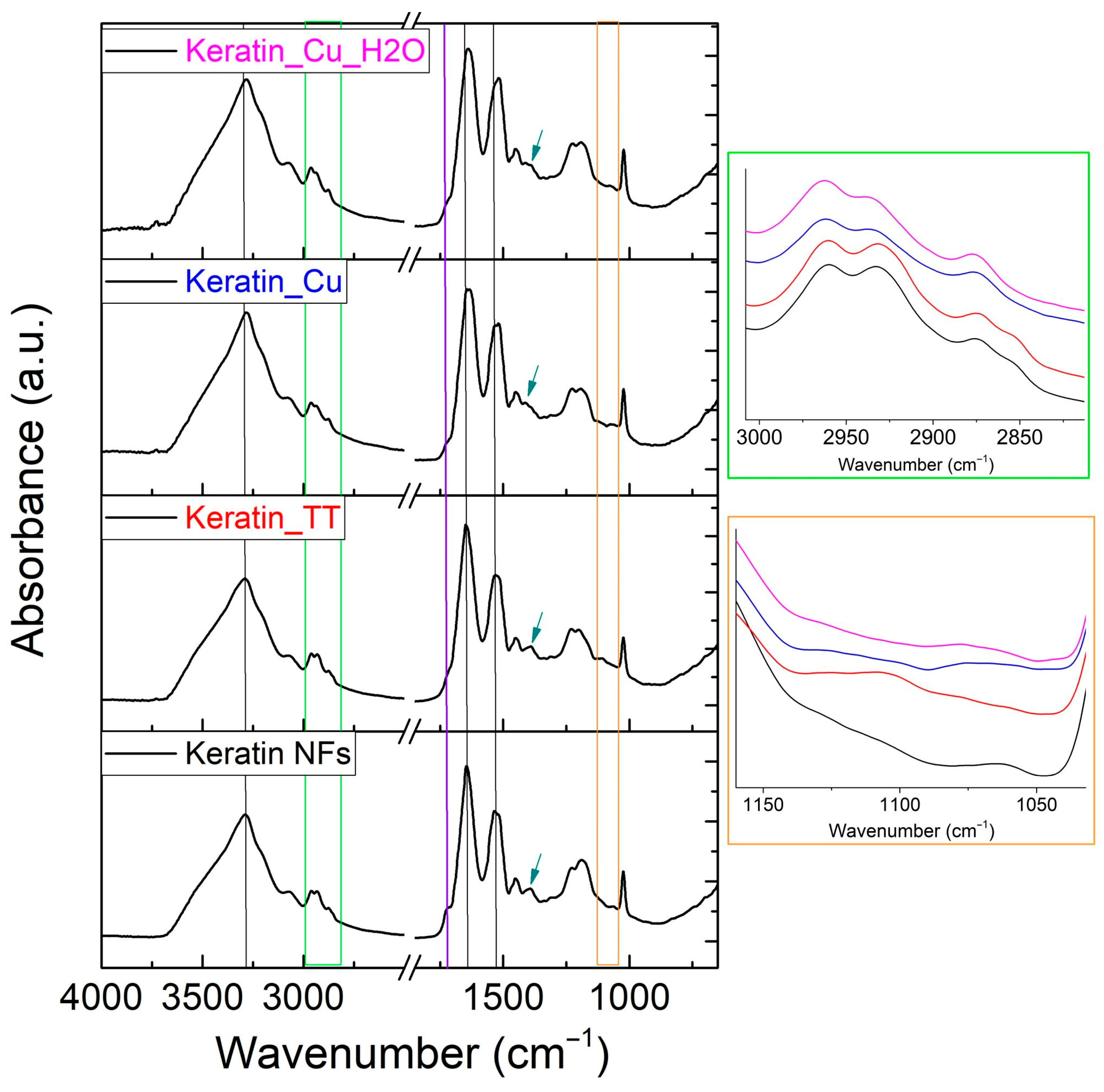
3.2. Compositional and Physical–Chemical Characterization
3.3. Antibacterial Tests
3.4. In Vitro Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared spectroscopy |
| CMFDA | 5-chloromethyl-fluorescein diacetate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified eagle medium |
| DSC | Differential scanning calorimetry |
| DTG | Derivative thermogravimetry |
| ECM | Extracellular matrix |
| EDS | Glutamic acid–serine |
| EDX | X-ray energy dispersive spectroscopy |
| FBS | Fetal bovine serum |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| LDV | Leucine–aspartic acid–valine |
| NFs | Nanofibers |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| RGD | Arginine–glycine–aspartic acid |
| RH | Relative humidity |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TCP | Tissue culture plate |
| TGA | Thermogravimetric analysis |
| TT | Thermal treatment |
References
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Laurenti, E.; Bracco, P.; Cecone, C.; La Parola, V.; Vineis, C.; Testa, M.L. Antibacterial Properties of Functionalized Cellulose Extracted from Deproteinized Soybean Hulls. Cellulose 2023, 30, 7805–7824. [Google Scholar] [CrossRef]
- Chaoui, L.; Mhand, R.; Mellouki, F.; Rhallabi, N. Contamination of the Surfaces of a Health Care Environment by Multidrug-Resistant (MDR) Bacteria. Int. J. Microbiol. 2019, 2019, 3236526. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Antimicrobial Resistance (2023). Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=Antimicrobial%20resistance%20(AMR)%20is%20one,4.95%20million%20deaths%20(1) (accessed on 15 May 2024).
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A Naturally Inspired Antibiotic to Target Multidrug-Resistant Pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-Guided Discovery of Antibiotics that Inhibit Peptidoglycan Remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef]
- Agnihotri, S.; Dhiman, N.K. Development of Nano-Antimicrobial Biomaterials for Biomedical Applications. In Advances in Biomaterials for Biomedical Applications, Advanced Structured Materials 66; Tripathi, A., Melo, J.S., Eds.; Springer Nature: Singapore, 2017; Volume 66, pp. 479–545. ISBN 9789811033285. [Google Scholar]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-mediated HMSC Behavior on Electrospun Scaffolds for Skeletal Muscle Regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Musale, S.; Panzade, P.; Paiva-Santos, A.C.; Sonwane, P.; Madibone, M.; Choundhe, P.; Giram, P.; Cavalu, S. Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications. Nanomaterials 2022, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.B.; Pawar, R.; Vitore, J.; Kulkarni, D.; Musale, S.; Giram, P.S. Natural and Synthetic Functional Materials for Broad Spectrum Applications in Antimicrobials, Antivirals and Cosmetics. Polym. Adv. Technol. 2021, 32, 4204–4222. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Sierra, A.A.; Bedford, N.M.; Lyon, W.J.; Gruner, W.E.; Mirau, P.A.; Naik, R.R. Keratin-Based Antimicrobial Textiles, Films, and Nanofibers. J. Mater. Chem. B 2013, 1, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.; Balu, S.K.; Jeevanandam, J.; Muthalagu, M. Emerging Nanomaterials for Antibacterial Textile Fabrication. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1355–1382. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-Based Biomaterials for Bone and Cartilage Tissue Engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Hsieh, J.H.; Li, C.; Chang, Y.K.; Yang, C.C. Dissolution of Cu Nanoparticles and Antibacterial Behaviors of TaN-Cu Nanocomposite Thin Films. Thin Solid Films 2009, 517, 4956–4960. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Ul Hasan, M.M. Investigations into the Antibacterial Behavior of Copper Nanoparticles against Escherichia Coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Cochis, A.; Ferraris, S.; Sorrentino, R.; Azzimonti, B.; Novara, C.; Geobaldo, F.; Truffa Giachet, F.; Vineis, C.; Varesano, A.; Sayed Abdelgeliel, A.; et al. Silver-Doped Keratin Nanofibers Preserve a Titanium Surface from Biofilm Contamination and Favor Soft-Tissue Healing. J. Mater. Chem. B 2017, 5, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Putting Copper into Action: Copper-impregnated Products with Potent Biocidal Activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2015, 8, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Xu, K.; Zhong, H.; Yang, Y.; Jin, S.; Yang, K.; Qi, X. Biological Applications of Copper-Containing Materials. Bioact. Mater. 2021, 6, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Boda, S.K.; Teusink, M.J.; Shuler, F.D.; Li, X.; Xie, J. Binary Doping of Strontium and Copper Enhancing Osteogenesis and Angiogenesis of Bioactive Glass Nanofibers While Suppressing Osteoclast Activity. ACS Appl. Mater. Interfaces 2017, 9, 24484–24496. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.R.; Zhang, W.X.; Wei, Y.N.; Li, Y.; Fei, T.; Shu, Y.; Wang, J.H. Ionic Liquids Enable the Preparation of a Copper-Loaded Gel with Transdermal Delivery Function for Wound Dressings. Biomater. Sci. 2022, 10, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper Nanoparticles Promote Rapid Wound Healing in Acute Full Thickness Defect via Acceleration of Skin Cell Migration, Proliferation, and Neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, V.A.E.; Shaikh, I.V. Polysaccharides: Sustainable Green-Composite Biomaterials. In Encyclopedia of Green Materials; Baskar, C., Ramakrishna, S., La Rosa, D., Eds.; Springer Nature: Singapore, 2022; pp. 1–11. [Google Scholar]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Applicability of New Sustainable and Efficient Alginate-Based Composites for Critical Raw Materials Recovery: General Composites Fabrication Optimization and Adsorption Performance Evaluation. Chem. Eng. J. 2022, 446, 137245. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Santuchi da Cunha, A.; Sederino, V.; Fabbri, D.; Tummino, M.L.; Laurenti, E. Soybean Peroxidase Immobilised on Cellulose-Alginate Hydrogels for Removal of Recalcitrant Organic Pollutants in Water. React. Chem. Eng. 2023, 8, 1629–1637. [Google Scholar] [CrossRef]
- Salama, A.; Abou-Zeid, R.E.; Cruz-Maya, I.; Guarino, V. Soy Protein Hydrolysate Grafted Cellulose Nanofibrils with Bioactive Signals for Bone Repair and Regeneration. Carbohydr. Polym. 2020, 229, 115472. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Jedvert, K.; El Seoud, O.A. Engineering of Sustainable Biomaterial Composites from Cellulose and Silk Fibroin: Fundamentals and Applications. Int. J. Biol. Macromol. 2021, 167, 687–718. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guzmán, C.J.; Castro-Muñoz, R. A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes 2020, 8, 1376. [Google Scholar] [CrossRef]
- Vineis, C.; Cruz Maya, I.; Mowafi, S.; Varesano, A.; Sánchez Ramírez, D.O.; Abou Taleb, M.; Tonetti, C.; Guarino, V.; El-Sayed, H. Synergistic Effect of Sericin and Keratin in Gelatin Based Nanofibers for in Vitro Applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Cao, G.; Rong, M.Z.; Zhang, M.Q. Continuous High-Content Keratin Fibers with Balanced Properties Derived from Wool Waste. ACS Sustain. Chem. Eng. 2020, 8, 18148–18156. [Google Scholar] [CrossRef]
- Zhu, S.; Zeng, W.; Meng, Z.; Luo, W.; Ma, L.; Li, Y.; Lin, C.; Huang, Q.; Lin, Y.; Liu, X.Y. Using Wool Keratin as a Basic Resist Material to Fabricate Precise Protein Patterns. Adv. Mater. 2019, 31, 1900870. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Ramirez, D.O.; Vineis, C.; Cruz-Maya, I.; Tonetti, C.; Guarino, V.; Varesano, A. Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field. J. Funct. Biomater. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Ramsey, D.H.; Hou, Y.; Cong, L.; Mohan, A.; Bekhit, A.E.D.A. Wool Keratin as a Novel Alternative Protein: A Comprehensive Review of Extraction, Purification, Nutrition, Safety, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 643–687. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous Materials: Structures and Functions in Biomedical Applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, E.; Hamlet, S.; George, R.; Sharma, A.; Love, R.M. Biofunctional Approaches of Wool-Based Keratin for Tissue Engineering. J. Sci. Adv. Mater. Devices 2022, 7, 100398. [Google Scholar] [CrossRef]
- Ye, W.; Qin, M.; Qiu, R.; Li, J. Keratin-Based Wound Dressings: From Waste to Wealth. Int. J. Biol. Macromol. 2022, 211, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Shin, H.K.; Kim, B.S.; Kim, M.J.; Kim, I.S.; Park, B.Y.; Kim, H.Y. Effect of Discarded Keratin-Based Biocomposite Hydrogels on the Wound Healing Process in Vivo. Mater. Sci. Eng. C 2015, 55, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero, J.A.; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From Fabric to Tissue: Recovered Wool Keratin/Polyvinylpyrrolidone Biocomposite Fibers as Artificial Scaffold Platform. Mater. Sci. Eng. C 2020, 116, 111151. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Shibayama, M.; Tanabe, T.; Yamauchi, K. Preparation and Physicochemical Properties of Compression-Molded Keratin Films. Biomaterials 2004, 25, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun Polyurethane/Keratin/AgNP Biocomposite Mats for Biocompatible and Antibacterial Wound Dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Arab-Bafrani, Z.; Karimi, F.; Javid, N. Designing Hybrid Nanofibers Based on Keratin-Poly (Vinyl Alcohol) and Poly (Ɛ-Caprolactone) for Application as Wound Dressing. J. Ind. Text. 2022, 51, 1729S–1949S. [Google Scholar] [CrossRef]
- Sharma, L.A.; Love, R.M.; Ali, M.A.; Sharma, A.; Macari, S.; Avadhani, A.; Dias, G.J. Healing Response of Rat Pulp Treated with an Injectable Keratin Hydrogel. J. Appl. Biomater. Funct. Mater. 2017, 15, e244–e250. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Cruz Maya, I.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned Keratin Submicrometric-Fibers for Fibroblasts Guidance onto Nanogrooved Titanium Surfaces for Transmucosal Implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Ramirez, D.O.S.; Cruz-Maya, I.; Vineis, C.; Guarino, V.; Tonetti, C.; Varesano, A. Wool Keratin-Based Nanofibres—In Vitro Validation. Bioengineering 2021, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Mowafi, S.; El-Sayed, H. Production and Utilization of Keratin and Sericin-Based Electro-Spun Nanofibers: A Comprehensive Review. J. Nat. Fibers 2023, 20, 2192544. [Google Scholar] [CrossRef]
- Ferraris, S.; Truffa Giachet, F.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and Keratin Nanofibers on Titanium Surfaces Aimed at Driving Gingival Fibroblasts Alignment and Proliferation without Increasing Bacterial Adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenço, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and Antibacterial Efficacy of Graphene Oxide Nanocomposite Fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly Polydisperse Keratin Rich Nanofibers: Scaffold Design and in Vitro Characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, R.; Rahimi, M.K.; Abbasipour, M.; Brendjchi, A.H. Antibacterial Nanofibrous Scaffolds with Lowered Cytotoxicity Using Keratin Extracted from Quail Feathers. J. Bioact. Compat. Polym. 2016, 31, 60–71. [Google Scholar] [CrossRef]
- Freddi, G.; Arai, T.; Colonna, G.M.; Boschi, A.; Tsukada, M. Binding of Metal Cations to Chemically Modified Wool and Antimicrobial Properties of the Wool-Metal Complexes. J. Appl. Polym. Sci. 2001, 82, 3513–3519. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun Silver Nanoparticles-Embedded Feather Keratin/Poly(Vinyl Alcohol)/Poly(Ethylene Oxide) Antibacterial Composite Nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Varesano, A.; Vineis, C.; Tonetti, C.; Ramirez, D.; Mazzuchetti, G.; Ortelli, S.; Blosi, M.; Costa, A. Multifunctional Hybrid Nanocomposite Nanofibers Produced by Colloid Electrospinning from Water Solutions. Curr. Nanosci. 2014, 11, 41–48. [Google Scholar] [CrossRef]
- Aluigi, A.; Corbellini, A.; Rombaldoni, F.; Mazzuchetti, G. Wool-Derived Keratin Nanofiber Membranes for Dynamic Adsorption of Heavy-Metal Ions from Aqueous Solutions. Text. Res. J. 2013, 83, 1574–1586. [Google Scholar] [CrossRef]
- Figoli, A.; Ursino, C.; Sanchez Ramirez, D.O.; Carletto, R.A.; Tonetti, C.; Varesano, A.; De Santo, M.P.; Cassano, A.; Vineis, C. Fabrication of Electrospun Keratin Nanofiber Membranes for Air and Water Treatment. Polym. Eng. Sci. 2019, 59, 1472–1478. [Google Scholar] [CrossRef]
- Tummino, M.L.; Vineis, C.; Varesano, A.; Liotta, L.F.; Rigoletto, M.; Laurenti, E.; Deganello, F. Sr0.85Ce0.15Fe0.67Co0.33-XCuxO3 Perovskite Oxides: Effect of B-Site Copper Codoping on the Physicochemical, Catalytic and Antibacterial Properties upon UV or Thermal Activation. Front. Environ. Eng. 2023, 2, 1249931. [Google Scholar] [CrossRef]
- Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2013.
- Varesano, A.; Vineis, C.; Tonetti, C.; Ramírez, D.O.S.; Mazzuchetti, G. Chemical and Physical Modifications of Electrospun Keratin Nanofibers Induced by Heating Treatments. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q. Greener Synthesis of Electrospun Collagen/Hydroxyapatite Composite Fibers with an Excellent Microstructure for Bone Tissue Engineering. Int. J. Nanomed. 2015, 10, 3203. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, D.; Li, J.; Li, L.; Li, W.; Cui, W.; Liu, K. Highly Efficient Extraction of Large Molecular-Weight Keratin from Wool in a Water/Ethanol Co-Solvent. Text. Res. J. 2020, 90, 1084–1093. [Google Scholar] [CrossRef]
- Yin, C.; Feng, L.; Zhang, N.; Cheng, Y. How Environmental Factors Affect the Structural Properties and Biofunctions of Keratin: A Molecular Dynamics Study. Mater. Today Commun. 2023, 34, 105254. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Khumalo, M.; Sithole, B.; Tesfaye, T.; Lekha, P. Valorization of Waste Chicken Feathers: Fabrication and Characterization of Novel Keratin Nanofiber Conduits for Potential Application in Peripheral Nerve Regeneration. J. Nanomater. 2022, 2022, 7080278. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Wang, Y.; Wang, Q. Second-Order Derivation Fourier Transform Infrared Spectral Analysis of Regenerated Wool Keratin Structural Changes. AATCC J. Res. 2022, 9, 43–48. [Google Scholar] [CrossRef]
- Ashtarinezhad, A.; Shirazi, F.H.; Vatanpour, H.; Mohamazadehasl, B.; Panahyab, A.; Nakhjavani, M. FTIR-Microspectroscopy Detection of Metronidazole Teratogenic Effects on Mice Fetus. Iran. J. Pharm. Res. 2014, 13, 101–111. [Google Scholar] [PubMed]
- Kumar, S.; Reena; Chaudhary, S.; Sweety; Jain, D.C. Vibrational Studies of Different Human Body Disorders Using FTIR Spectroscopy. Open J. Appl. Sci. 2014, 4, 103–129. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kabutare, Y.H.; Ghosh, P. Dual Crosslinked Keratin-Alginate Fibers Formed via Ionic Complexation of Amide Networks with Improved Toughness for Assembling into Braids. Polym. Test. 2020, 81, 106286. [Google Scholar] [CrossRef]
- Baddiel, C.B. Structure and Reactions of Human Hair Keratin: An Analysis by Infrared Spectroscopy. J. Mol. Biol. 1968, 38, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Selmin, F.; Cilurzo, F.; Aluigi, A.; Franzè, S.; Minghetti, P. Regenerated Keratin Membrane to Match the In Vitro Drug Diffusion through Human Epidermis. Res. Pharma Sci. 2012, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Heliopoulos, N.S.; Papageorgiou, S.K.; Galeou, A.; Favvas, E.P.; Katsaros, F.K.; Stamatakis, K. Effect of Copper and Copper Alginate Treatment on Wool Fabric. Study of Textile and Antibacterial Properties. Surf. Coatings Technol. 2013, 235, 24–31. [Google Scholar] [CrossRef]
- Gazioglu Ruzgar, D.; Altun Kurtoglu, S.; Bhullar, S.K. A Study on Extraction and Characterization of Keratin Films and Nanofibers from Waste Wool Fiber. J. Nat. Fibers 2020, 17, 427–436. [Google Scholar] [CrossRef]
- Tu, H.; Yu, R.; Lin, Z.; Zhang, L.; Lin, N.; Yu, W.D.; Liu, X.Y. Programing Performance of Wool Keratin and Silk Fibroin Composite Materials by Mesoscopic Molecular Network Reconstruction. Adv. Funct. Mater. 2016, 26, 9032–9043. [Google Scholar] [CrossRef]
- Belukhina, O.; Milasiene, D.; Ivanauskas, R. Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide. Materials 2021, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Fonfria, V.A. Infrared Difference Spectroscopy of Proteins: From Bands to Bonds. Chem. Rev. 2020, 120, 3466–3576. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, Y.; Yao, J.; Shen, Y.; Wu, H.; Li, J.; Yang, M. Characterization of the Keratin/Polyamide 6 Composite Fiber’s Structure and Performance Prepared by the Optimized Spinning Process Based on the Rheological Analysis. Int. J. Biol. Macromol. 2022, 222, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Pielesz, A.; Wlochowicz, A.; Biniaś, W. The Evaluation of Structural Changes in Wool Fibre Keratin Treated with Azo Dyes by Fourier Transform Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Haladu, S.A.; Al-Hamouz, O.C.S.; Ali, S.A. Adsorption of Cd2+ and Cu2+ Ions from Aqueous Solutions by a Cross-Linked Polysulfonate–Carboxylate Resin. Arab. J. Chem. 2019, 12, 2597–2607. [Google Scholar] [CrossRef]
- Sun, J.; Monreal Santiago, G.; Yan, F.; Zhou, W.; Rudolf, P.; Portale, G.; Kamperman, M. Bioinspired Processing of Keratin into Upcycled Fibers through PH-Induced Coacervation. ACS Sustain. Chem. Eng. 2023, 11, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Zinigrad, M.; Sobolev, A.; Nisnevitch, M. Keratin Biomembranes as a Model for Studying Onychomycosis. Int. J. Mol. Sci. 2020, 21, 3512. [Google Scholar] [CrossRef] [PubMed]
- Bubela, B.; Powell, T.G. Effect of Copper on the Composition of Bacterial Cell Wall Peptides’). Zentralblatt Bakteriol. Parasitenkunde Infekt. Hyg. Zweite Naturwissenschaftliche Abteilung Allg. Landwirtsch. Tech. Mikrobiol. 1973, 128, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Grass, G. Escherichia Coli Mechanisms of Copper Homeostasis in a Changing Environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Fowler, L.; Engqvist, H.; Öhman-Mägi, C. Effect of Copper Ion Concentration on Bacteria and Cells. Materials 2019, 12, 3798. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin–Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Muszyński, S.; Ognik, K.; Dobrowolski, P.; Kwiecień, M.; Juśkiewicz, J.; Chocyk, D.; Świetlicki, M.; Blicharski, T.; Gładyszewska, B. Comparison of the Effect of Dietary Copper Nanoparticles with Copper (II) Salt on Bone Geometric and Structural Parameters as Well as Material Characteristics in a Rat Model. J. Trace Elem. Med. Biol. 2017, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper Induces Oxidative Stress and Apoptosis through Mitochondria-Mediated Pathway in Chicken Hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Zhu, Y.; Wang, X.; Li, M.; Lin, Z.; Hu, X.; Zhang, Y.; Yin, Q.; Xia, H.; et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle Cytotoxicity Depends on Intracellular Solubility: Comparison of Stabilized Copper Metal and Degradable Copper Oxide Nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Colombo, G.; Chirico, G.; Capitani, G.; Dalle-Donne, I.; Mantecca, P. Biological Mechanism of Cell Oxidative Stress and Death during Short-Term Exposure to Nano CuO. Sci. Rep. 2023, 13, 2326. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zheng, Y.; Xi, T.; Zhang, C.; Song, W.; Burugapalli, K.; Yang, H.; Ma, Y. Concentration-Dependent Cytotoxicity of Copper Ions on Mouse Fibroblasts in Vitro: Effects of Copper Ion Release from TCu380A vs TCu220C Intra-Uterine Devices. Biomed. Microdevices 2012, 14, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Liu, S.-T.; Lin, W.-R.; Lin, C.-K.; Huang, S.-M. The Mechanisms Underlying the Cytotoxic Effects of Copper Via Differentiated Embryonic Chondrocyte Gene 1. Int. J. Mol. Sci. 2019, 20, 5225. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Fazly Bazzaz, B.S.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The Role of Nanotechnology in Combating Biofilm-Based Antibiotic Resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Lugo-Uribe, L.E.; Pérez-Alvarez, M.; Tavizón, S.F.; Santillán, G. de J.S. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021, 13, 1694. [Google Scholar] [CrossRef] [PubMed]
- Eslaminezhad, S.; Moradi, F.; Hojjati, M.R. Evaluation of the Wound Healing Efficacy of New Antibacterial Polymeric Nanofiber Based on Polyethylene Oxide Coated with Copper Nanoparticles and Defensin Peptide: An in-Vitro to in-Vivo Assessment. Heliyon 2024, 10, e29542. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, M.S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In Vitro and in Vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Vergara-Figueroa, J.; Alejandro-Martin, S.; Cerda-Leal, F.; Gacitúa, W. Dual Electrospinning of a Nanocomposites Biofilm: Potential Use as an Antimicrobial Barrier. Mater. Today Commun. 2020, 25, 101671. [Google Scholar] [CrossRef]
- Shalaby, T.; Hamad, H.; Ibrahim, E.; Mahmoud, O.; Al-Oufy, A. Electrospun Nanofibers Hybrid Composites Membranes for Highly Efficient Antibacterial Activity. Ecotoxicol. Environ. Saf. 2018, 162, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Sen, R.K.; Patel, M.; Shruti; Dwivedi, N.; Singh, S.; Kumar, P.; Chouhan, M.; Yadav, A.K.; Mondal, D.P.; et al. Development of Copper Impregnated Bio-Inspired Hydrophobic Antibacterial Nanocoatings for Textiles. Colloids Surfaces B Biointerfaces 2022, 220, 112913. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Keratin NFs | Keratin_TT | Keratin_Cu |
|---|---|---|---|
| TGA residue (wt%) | 25 | 12 | 24 |
| DTG peak 1 (°C) | 213 | 222 | 266 |
| DTG peak 2 (°C) | 286 | 300 | 314 |
| DSC peak 1 (°C) | 225 | 229 | 276 |
| DSC peak 2 (°C) | 300 | 310 | 331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummino, M.L.; Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Keratin/Copper Complex Electrospun Nanofibers for Antibacterial Treatments: Property Investigation and In Vitro Response. Materials 2024, 17, 2435. https://doi.org/10.3390/ma17102435
Tummino ML, Cruz-Maya I, Varesano A, Vineis C, Guarino V. Keratin/Copper Complex Electrospun Nanofibers for Antibacterial Treatments: Property Investigation and In Vitro Response. Materials. 2024; 17(10):2435. https://doi.org/10.3390/ma17102435
Chicago/Turabian StyleTummino, Maria Laura, Iriczalli Cruz-Maya, Alessio Varesano, Claudia Vineis, and Vincenzo Guarino. 2024. "Keratin/Copper Complex Electrospun Nanofibers for Antibacterial Treatments: Property Investigation and In Vitro Response" Materials 17, no. 10: 2435. https://doi.org/10.3390/ma17102435









