Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications
Abstract
:1. Introduction
2. Synthesis of HA Hydrogels
2.1. Chemical Cross-Linking
2.1.1. Carbodiimide Cross-Linking
2.1.2. Diisocyanate Cross-Linking
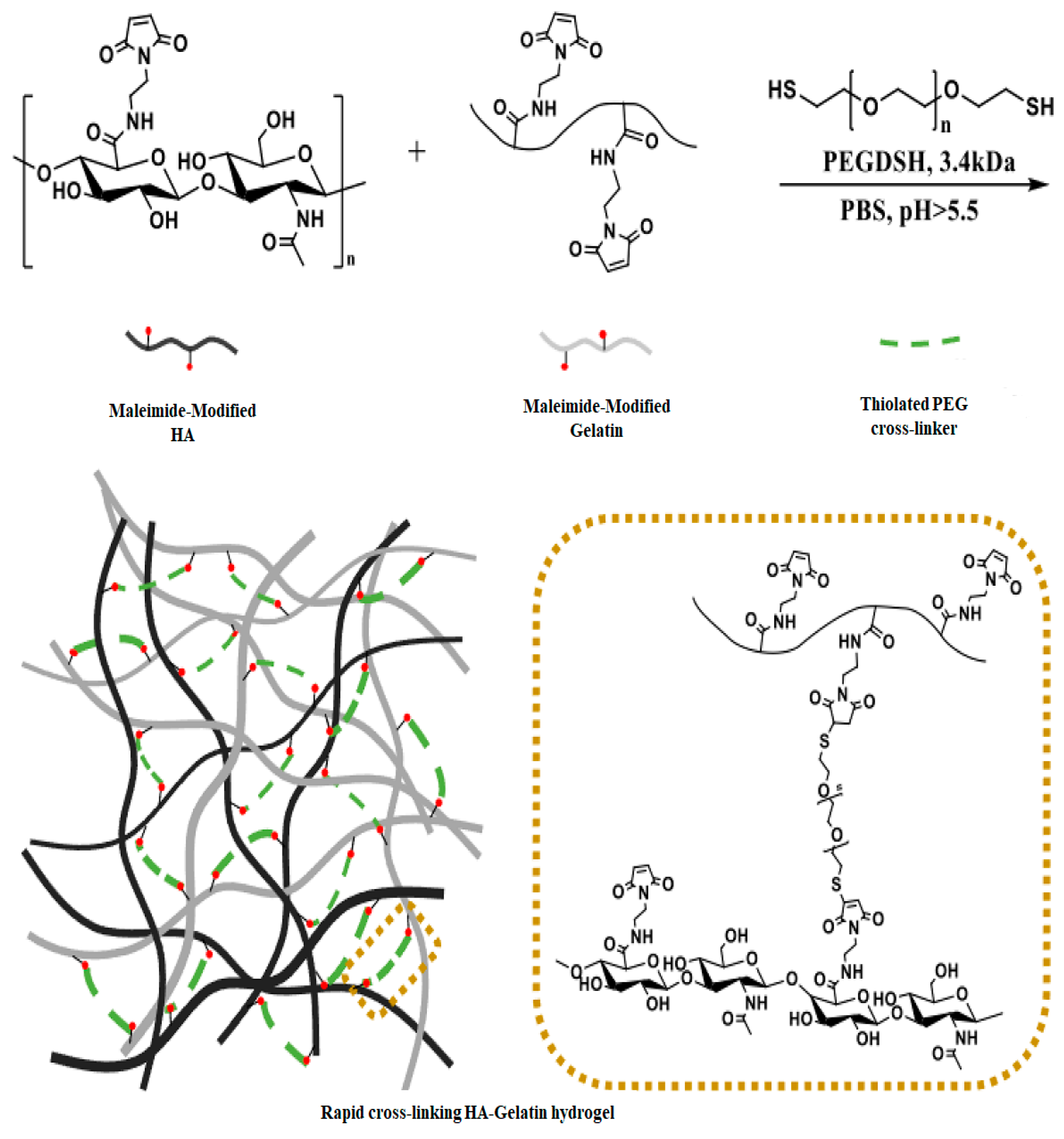
2.1.3. Michael Addition
2.1.4. Esterification
2.1.5. Diels–Alder (D-A) Cross-Linking
2.1.6. Photo Cross-Linking
2.1.7. Miscellaneous Cross-Linking Methods
Thiol-ene Click
Etherification
Amidation
Hydrazone Linkage
2.1.8. Identification and Quantification of Functionalized HA
Fourier-Transform Infrared Spectroscopy (FTIR)
Proton Nuclear Magnetic Resonance (1H NMR)
Carbon-13 Nuclear Magnetic Resonance (13C NMR)
Titration
2.2. Physical Cross-Linking
2.2.1. Temperature-Induced Gelation
2.2.2. Covalent Augmentation
2.2.3. Freeze–Thawing
2.3. Enzymatic Cross-Linking
3. Techniques Used to Investigate the Properties of HA Hydrogels
3.1. Rheological Analysis
3.2. Swelling Behavior
3.3. Morphology Examination
3.4. Thermal Analysis
4. Utilization of Hydrogels Based on HA
4.1. Tissue Engineering
4.2. Drug Delivery Systems
4.3. Wound Healing
4.4. Ophthalmology
4.5. Three-Dimensional Bioprinting
4.6. Three-Dimensional Culture and Disease Modeling
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholamali, I.; Yadollahi, M. Doxorubicin-Loaded Carboxymethyl Cellulose/Starch/ZnO Nanocomposite Hydrogel Beads as an Anticancer Drug Carrier Agent. Int. J. Biol. Macromol. 2020, 160, 724–735. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and Characterization of Antibacterial Carboxymethyl Cellulose/ZnO Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and Characterization of Antibacterial Carboxymethylcellulose/CuO Bio-Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef]
- Zheng, Y.; Baidya, A.; Annabi, N. Molecular Design of an Ultra-Strong Tissue Adhesive Hydrogel with Tunable Multifunctionality. Bioact. Mater. 2023, 29, 214–229. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, Alginate, Hyaluronic Acid and Other Novel Multifunctional Hydrogel Dressings for Wound Healing: A Review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2021, 7, 91–114. [Google Scholar] [CrossRef]
- Gholamali, I.; Hosseini, S.N.; Alipour, E.; Yadollahi, M. Preparation and Characterization of Oxidized Starch/CuO Nanocomposite Hydrogels Applicable in a Drug Delivery System. Starch—Stärke 2019, 71, 1800118. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and Evaluation of Alginate, Gelatin, and Hyaluronic Acid Hybrid Hydrogels for Tissue Engineering Applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- De Zheng, B.; Xiao, M.T. Polysaccharide-Based Hydrogel with Photothermal Effect for Accelerating Wound Healing. Carbohydr. Polym. 2023, 299, 120228. [Google Scholar] [CrossRef]
- Khandan-Nasab, N.; Mahdipour, E.; Askarian, S.; Kalantari, M.R.; Ramezanian, N.; Kazemi Oskuee, R. Design and Characterization of Adipose-Derived Mesenchymal Stem Cell Loaded Alginate/Pullulan/Hyaluronic Acid Hydrogel Scaffold for Wound Healing Applications. Int. J. Biol. Macromol. 2023, 241, 124556. [Google Scholar] [CrossRef]
- Sekar, M.P.; Suresh, S.; Zennifer, A.; Sethuraman, S.; Sundaramurthi, D. Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 3134–3159. [Google Scholar] [CrossRef]
- Gholamali, I.; Asnaashariisfahani, M.; Alipour, E. Silver Nanoparticles Incorporated in PH-Sensitive Nanocomposite Hydrogels Based on Carboxymethyl Chitosan-Poly (Vinyl Alcohol) for Use in a Drug Delivery System. Regen. Eng. Transl. Med. 2020, 6, 138–153. [Google Scholar] [CrossRef]
- Agarwal, G.; Agiwal, S.; Srivastava, A. Hyaluronic Acid Containing Scaffolds Ameliorate Stem Cell Function for Tissue Repair and Regeneration. Int. J. Biol. Macromol. 2020, 165, 388–401. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Li, D.; Li, X.; Liu, Y. From Crosslinking Strategies to Biomedical Applications of Hyaluronic Acid-Based Hydrogels: A Review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef]
- Kikani, T.; Dave, S.; Thakore, S. Functionalization of Hyaluronic Acid for Development of Self-Healing Hydrogels for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2023, 242, 124950. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In Situ Formed Collagen-Hyaluronic Acid Hydrogel as Biomimetic Dressing for Promoting Spontaneous Wound Healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging Roles of Hyaluronic Acid Bioscaffolds in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rai, A.K.; Prakash Tewari, R. Recent Advancement in Hyaluronic Acid-Based Hydrogel for Biomedical Engineering Application: A Mini-Review. Mater. Today Proc. 2023, 78, 138–144. [Google Scholar] [CrossRef]
- Yeom, J.; Bhang, S.H.; Kim, B.-S.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Park, J.K.; Hahn, S.K. Effect of Cross-Linking Reagents for Hyaluronic Acid Hydrogel Dermal Fillers on Tissue Augmentation and Regeneration. Bioconjug. Chem. 2010, 21, 240–247. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef]
- Li, R.; Cai, Z.; Li, Z.; Zhang, Q.; Zhang, S.; Deng, L.; Lu, L.; Li, L.; Zhou, C. Synthesis of In-Situ Formable Hydrogels with Collagen and Hyaluronan through Facile Michael Addition. Mater. Sci. Eng. C 2017, 77, 1035–1043. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, P.; Huangshan, L.; Hu, B.-H.; Messersmith, P.B. Improved Method for Synthesis of Cysteine Modified Hyaluronic Acid for in Situ Hydrogel Formation. Chem. Commun. 2015, 51, 9662–9665. [Google Scholar] [CrossRef]
- Goodarzi, K.; Rao, S.S. Hyaluronic Acid-Based Hydrogels to Study Cancer Cell Behaviors. J. Mater. Chem. B 2021, 9, 6103–6115. [Google Scholar] [CrossRef]
- Yoo, K.M.; Murphy, S.V.; Skardal, A. A Rapid Crosslinkable Maleimide-Modified Hyaluronic Acid and Gelatin Hydrogel Delivery System for Regenerative Applications. Gels 2021, 7, 13. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Q.; Liang, K.; Zhou, D.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y.; Xiao, P. Photopolymerizable Thiol-Acrylate Maleiated Hyaluronic Acid/Thiol-Terminated Poly(Ethylene Glycol) Hydrogels as Potential in-Situ Formable Scaffolds. Int. J. Biol. Macromol. 2018, 119, 270–277. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and Characterization of Hyaluronic Acid Hydrogels Crosslinked Using a Solvent-Free Process for Potential Biomedical Applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Bedini, E.; Cassese, E.; D’Agostino, A.; Cammarota, M.; Frezza, M.A.; Lepore, M.; Portaccio, M.; Schiraldi, C.; La Gatta, A. Self-Esterified Hyaluronan Hydrogels: Advancements in the Production with Positive Implications in Tissue Healing. Int. J. Biol. Macromol. 2023, 236, 123873. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, S.; Zhao, J.; Zhu, H.; Pan, X.; Zhao, B.; Sun, Z.; Li, N.; Hou, X. Development of Injectable in Situ Hydrogels Based on Hyaluronic Acid via Diels-Alder Reaction for Their Antitumor Activities Studies. Int. J. Biol. Macromol. 2024, 262, 129642. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 204173141772646. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, Z.-H.; Kuo, C.-Y.; Chen, J.-P. Photo-Crosslinked Hyaluronic Acid/Carboxymethyl Cellulose Composite Hydrogel as a Dural Substitute to Prevent Post-Surgical Adhesion. Int. J. Mol. Sci. 2022, 23, 6177. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In Situ-Forming Hyaluronic Acid Hydrogel through Visible Light-Induced Thiol-Ene Reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Piperno, A.; Mahfoudh, N.; Schurigt, U.; Schultheis, M.; Mineo, P.G.; Schirmeister, T.; Scala, A.; Grassi, G. A Hyaluronic Acid–Pentamidine Bioconjugate as a Macrophage Mediated Drug Targeting Delivery System for the Treatment of Leishmaniasis. RSC Adv. 2015, 5, 95545–95550. [Google Scholar] [CrossRef]
- Bokatyi, A.N.; Dubashynskaya, N.V.; Skorik, Y.A. Chemical Modification of Hyaluronic Acid as a Strategy for the Development of Advanced Drug Delivery Systems. Carbohydr. Polym. 2024, 337, 122145. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. PH-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Curcumin Delivery and Enhanced Cancer Therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Lin, R.; Cui, S.; Jing, X.; Coseri, S. Injectable Multifunctional Carboxymethyl Chitosan/Hyaluronic Acid Hydrogel for Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 249, 125801. [Google Scholar] [CrossRef]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Saeedi Garakani, S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Hashemi Motlagh, G. Injectable PNIPAM/Hyaluronic Acid Hydrogels Containing Multipurpose Modified Particles for Cartilage Tissue Engineering: Synthesis, Characterization, Drug Release and Cell Culture Study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater. 2018, 7, 1800225. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; D’Amora, U.; Raucci, M.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. A Combined Approach of Double Network Hydrogel and Nanocomposites Based on Hyaluronic Acid and Poly(Ethylene Glycol) Diacrylate Blend. Materials 2018, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Legay, L.; Budtova, T.; Buwalda, S. Hyaluronic Acid Aerogels Made Via Freeze–Thaw-Induced Gelation. Biomacromolecules 2023, 24, 4502–4509. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Khan, F.; Heo, S.Y.; Kim, T.H.; Kim, Y.M.; Yi, M.; Jung, W.K. Multifunctional Dual Cross-Linked Poly (Vinyl Alcohol)/Methacrylate Hyaluronic Acid/Chitooligosaccharide-Sinapic Acid Wound Dressing Hydrogel. Int. J. Biol. Macromol. 2022, 222, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Sakai, S.; Taya, M. Characterization of Encapsulated Cells within Hyaluronic Acid and Alginate Microcapsules Produced via Horseradish Peroxidase-Catalyzed Crosslinking. J. Biomater. Sci. Polym. Ed. 2019, 30, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, X.; Zoetebier, B.; Dijkstra, P.J.; Karperien, M. Enzymatic Co-Crosslinking of Star-Shaped Poly(Ethylene Glycol) Tyramine and Hyaluronic Acid Tyramine Conjugates Provides Elastic Biocompatible and Biodegradable Hydrogels. Bioact. Mater. 2023, 20, 53–63. [Google Scholar] [CrossRef]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue Adhesive Hyaluronic Acid Hydrogels for Sutureless Stem Cell Delivery and Regeneration of Corneal Epithelium and Stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H.H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Lee, K.-J.; Park, M.H.; Lee, S.-H.; Hahn, S.K.; Kim, S.; Kim, B.-S. Mechanical Properties and Degradation Behaviors of Hyaluronic Acid Hydrogels Cross-Linked at Various Cross-Linking Densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Bahari Javan, N.; Montazeri, H.; Rezaie Shirmard, L.; Jafary Omid, N.; Barbari, G.R.; Amini, M.; Ghahremani, M.H.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F. Preparation, Characterization and in Vivo Evaluation of a Combination Delivery System Based on Hyaluronic Acid/Jeffamine Hydrogel Loaded with PHBV/PLGA Blend Nanoparticles for Prolonged Delivery of Teriparatide. Eur. J. Pharm. Sci. 2017, 101, 167–181. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Wu, H.-C.; Sun, J.-S.; Dong, G.-C.; Wang, T.-W. Injectable and Thermoresponsive Self-Assembled Nanocomposite Hydrogel for Long-Term Anticancer Drug Delivery. Langmuir 2013, 29, 3721–3729. [Google Scholar] [CrossRef]
- Zamboni, F.; Ryan, E.; Culebras, M.; Collins, M.N. Labile Crosslinked Hyaluronic Acid via Urethane Formation Using Bis(β-Isocyanatoethyl) Disulphide with Tuneable Physicochemical and Immunomodulatory Properties. Carbohydr. Polym. 2020, 245, 116501. [Google Scholar] [CrossRef]
- Voss, K.; Falke, K.; Bernsdorf, A.; Grabow, N.; Kastner, C.; Sternberg, K.; Minrath, I.; Eickner, T.; Wree, A.; Schmitz, K.-P.; et al. Development of a Novel Injectable Drug Delivery System for Subconjunctival Glaucoma Treatment. J. Control. Release 2015, 214, 1–11. [Google Scholar] [CrossRef]
- Cao, W.; Sui, J.; Ma, M.; Xu, Y.; Lin, W.; Chen, Y.; Man, Y.; Sun, Y.; Fan, Y.; Zhang, X. The Preparation and Biocompatible Evaluation of Injectable Dual Crosslinking Hyaluronic Acid Hydrogels as Cytoprotective Agents. J. Mater. Chem. B 2019, 7, 4413–4423. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef]
- Szarpak, A.; Auzély-Velty, R. Hyaluronic Acid Single-Network Hydrogel with High Stretchable and Elastic Properties. Carbohydr. Polym. 2023, 320, 121212. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder Reaction in Polymer Chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. Eur. J. Org. Chem. 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Brasca, R.; Kneeteman, M.N.; Mancini, P.M.E.; Fabian, W.M.F. Theoretical Explanation of the Regioselectivity of Polar Cycloaddition Reactions between Furan Derivatives and Danishefsky’s Diene. J. Mol. Struct. Theochem 2009, 911, 124–131. [Google Scholar] [CrossRef]
- Qiu, Y. Substituent Effects in the Diels–Alder Reactions of Butadienes, Cyclopentadienes, Furans and Pyroles with Maleic Anhydride. J. Phys. Org. Chem. 2015, 28, 370–376. [Google Scholar] [CrossRef]
- Sahrane, M.; Marakchi, K.; Ghailane, R. Theoretical Study of the Diels–Alder Reaction of 3-Bromo-1-Phenylprop-2-Ynone with Furan and 2-Methylfuran. Theor. Chem. Acc. 2021, 140, 108. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Joo, S.-B.; Gulfam, M.; Jo, S.-H.; Jo, Y.-J.; Vu, T.T.; Park, S.-H.; Gal, Y.-S.; Lim, K.T. Fast Absorbent and Highly Bioorthogonal Hydrogels Developed by IEDDA Click Reaction for Drug Delivery Application. Materials 2022, 15, 7128. [Google Scholar] [CrossRef]
- Gulfam, M.; Jo, S.-H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.-B.; Park, S.-H.; Lim, K.T. NIR-Degradable and Biocompatible Hydrogels Derived from Hyaluronic Acid and Coumarin for Drug Delivery and Bio-Imaging. Carbohydr. Polym. 2023, 303, 120457. [Google Scholar] [CrossRef]
- Buckley, C.; Montgomery, T.R.; Szank, T.; Murray, B.A.; Quigley, C.; Major, I. Modification of Hyaluronic Acid to Enable Click Chemistry Photo-Crosslinking of Hydrogels with Tailorable Degradation Profiles. Int. J. Biol. Macromol. 2023, 240, 124459. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Peng, Y.; Zhang, S.; Zhang, Y.; Min, P. Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels 2022, 8, 609. [Google Scholar] [CrossRef]
- Li, R.; Sun, Y.; Cai, Z.; Li, Y.; Sun, J.; Bi, W.; Yang, F.; Zhou, Q.; Ye, T.; Yu, Y. Highly Bioactive Peptide-HA Photo-Crosslinking Hydrogel for Sustained Promoting Bone Regeneration. Chem. Eng. J. 2021, 415, 129015. [Google Scholar] [CrossRef]
- Wang, G.; Cao, X.; Dong, H.; Zeng, L.; Yu, C.; Chen, X. A Hyaluronic Acid Based Injectable Hydrogel Formed via Photo-Crosslinking Reaction and Thermal-Induced Diels-Alder Reaction for Cartilage Tissue Engineering. Polymers 2018, 10, 949. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Li, T.; Zhang, L.; Azhar, U.; Ma, J.; Zhai, C.; Zong, C.; Zhang, S. Cytocompatible and Non-Fouling Zwitterionic Hyaluronic Acid-Based Hydrogels Using Thiol-Ene “Click” Chemistry for Cell Encapsulation. Carbohydr. Polym. 2020, 236, 116021. [Google Scholar] [CrossRef]
- Korogiannaki, M.; Zhang, J.; Sheardown, H. Surface Modification of Model Hydrogel Contact Lenses with Hyaluronic Acid via Thiol-Ene “Click” Chemistry for Enhancing Surface Characteristics. J. Biomater. Appl. 2017, 32, 446–462. [Google Scholar] [CrossRef]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival Injectable Dendrimer-Dexamethasone Gel for the Treatment of Corneal Inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez Martínez, V.; Ruiz-Rubio, L.; Pérez González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible Hyaluronic Acid-Divinyl Sulfone Injectable Hydrogels for Sustained Drug Release with Enhanced Antibacterial Properties against Staphylococcus Aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef]
- Lai, J.Y. Relationship between Structure and Cytocompatibility of Divinyl Sulfone Cross-Linked Hyaluronic Acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef]
- Magnani, A.; Rappuoli, R.; Lamponi, S.; Barbucci, R. Novel Polysaccharide Hydrogels: Characterization and Properties. Polym. Adv. Technol. 2000, 11, 488–495. [Google Scholar] [CrossRef]
- Cui, N.; Qian, J.; Zhao, N.; Wang, H. Functional Hyaluronic Acid Hydrogels Prepared by a Novel Method. Mater. Sci. Eng. C 2014, 45, 573–577. [Google Scholar] [CrossRef]
- Mozipo, E.A.; Galindo, A.N.; Khachatourian, J.D.; Harris, C.G.; Dorogin, J.; Spaulding, V.R.; Ford, M.R.; Singhal, M.; Fogg, K.C.; Hettiaratchi, M.H. Statistical Optimization of Hydrazone-Crosslinked Hyaluronic Acid Hydrogels for Protein Delivery. J. Mater. Chem. B 2024, 12, 2523–2536. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.; Wu, C. Injectable and Body Temperature Sensitive Hydrogels Based on Chitosan and Hyaluronic Acid for PH Sensitive Drug Release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Luo, J.; Liu, C.; Wu, J.; Zhao, D.; Lin, L.; Fan, H.; Sun, Y. In Situ Forming Gelatin/Hyaluronic Acid Hydrogel for Tissue Sealing and Hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 790–797. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl Chitosan Microspheres Loaded Hyaluronic Acid/Gelatin Hydrogels for Controlled Drug Delivery and the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612. [Google Scholar] [CrossRef]
- Barroso, N.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L. Self-Healable Hyaluronic Acid/Chitosan Polyelectrolyte Complex Hydrogels and Multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Vu, T.T.; Gulfam, M.; Jo, S.H.; Rizwan, A.; Joo, S.B.; Lee, B.; Park, S.H.; Lim, K.T. The Effect of Molecular Weight and Chemical Structure of Cross-Linkers on the Properties of Redox-Responsive Hyaluronic Acid Hydrogels. Int. J. Biol. Macromol. 2023, 238, 124285. [Google Scholar] [CrossRef]
- Wende, F.J.; Gohil, S.; Mojarradi, H.; Gerfaud, T.; Nord, L.I.; Karlsson, A.; Boiteau, J.-G.; Kenne, A.H.; Sandström, C. Determination of Substitution Positions in Hyaluronic Acid Hydrogels Using NMR and MS Based Methods. Carbohydr. Polym. 2016, 136, 1348–1357. [Google Scholar] [CrossRef]
- Jo, Y.J.; Gulfam, M.; Jo, S.H.; Gal, Y.S.; Oh, C.W.; Park, S.H.; Lim, K.T. Multi-Stimuli Responsive Hydrogels Derived from Hyaluronic Acid for Cancer Therapy Application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef]
- Rampratap, P.; Lasorsa, A.; Perrone, B.; van der Wel, P.C.A.; Walvoort, M.T.C. Production of Isotopically Enriched High Molecular Weight Hyaluronic Acid and Characterization by Solid-State NMR. Carbohydr. Polym. 2023, 316, 121063. [Google Scholar] [CrossRef]
- Scott, J.E.; Heatley, F. Biological Properties of Hyaluronan in Aqueous Solution Are Controlled and Sequestered by Reversible Tertiary Structures, Defined by NMR Spectroscopy. Biomacromolecules 2002, 3, 547–553. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Yuan, B.; Chen, X. An Injectable Hyaluronic Acid/PEG Hydrogel for Cartilage Tissue Engineering Formed by Integrating Enzymatic Crosslinking and Diels–Alder “Click Chemistry”. Polym. Chem. 2014, 5, 1082–1090. [Google Scholar] [CrossRef]
- Seres, L.; Csapó, E.; Varga, N.; Juhász, Á. The Effect of Concentration, Temperature, and PH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels. Gels 2023, 9, 529. [Google Scholar] [CrossRef]
- Al-Amodi, A.; Hill, R.J. Streaming Potentials of Hyaluronic Acid Hydrogel Films. Langmuir 2022, 38, 13370–13381. [Google Scholar] [CrossRef]
- Casey-Power, S.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. Hyaluronic Acid: Its Versatile Use in Ocular Drug Delivery with a Specific Focus on Hyaluronic Acid-Based Polyelectrolyte Complexes. Pharmaceutics 2022, 14, 1479. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polym. Rev. 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Ijaz, U.; Sohail, M.; Usman Minhas, M.; Khan, S.; Hussain, Z.; Kazi, M.; Ahmed Shah, S.; Mahmood, A.; Maniruzzaman, M. Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers 2022, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-Sensitive Injectable Hydrogel Based on the Physical Mixing of Hyaluronic Acid and Pluronic F-127 for Sustained NSAID Delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Madau, M.; Le Cerf, D.; Dulong, V.; Picton, L. Hyaluronic Acid Functionalization with Jeffamine® M2005: A Comparison of the Thermo-Responsiveness Properties of the Hydrogel Obtained through Two Different Synthesis Routes. Gels 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Shokri, R.; Fuentes-Chandía, M.; Ai, J.; Habibi Roudkenar, M.; Reza Mahboubian, A.; Rad Malekshahi, M.; Nasser Ostad, S. A Thermo-Sensitive Hydrogel Composed of Methylcellulose/Hyaluronic Acid/Silk Fibrin as a Biomimetic Extracellular Matrix to Simulate Breast Cancer Malignancy. Eur. Polym. J. 2022, 176, 111421. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Farè, S. Crosslinking Strategies in Modulating Methylcellulose Hydrogel Properties. Soft Matter 2023, 19, 7869–7884. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Basu, A.; Maji, S.; Dutta, K.; Dewan, M.; Adhikary, A.; Maiti, T.K.; Chattopadhyay, D. Nanotailored Hyaluronic Acid Modified Methylcellulose as an Injectable Scaffold with Enhanced Physico-Rheological and Biological Aspects. Carbohydr. Polym. 2020, 237, 116146. [Google Scholar] [CrossRef] [PubMed]
- Mahboubian, A.; Vllasaliu, D.; Dorkoosh, F.A.; Stolnik, S. Temperature-Responsive Methylcellulose–Hyaluronic Hydrogel as a 3D Cell Culture Matrix. Biomacromolecules 2020, 21, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tavakoli, S.; Parvathaneni, R.P.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Dynamic Covalent Crosslinked Hyaluronic Acid Hydrogels and Nanomaterials for Biomedical Applications. Biomater. Sci. 2022, 10, 6399–6412. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified Hyaluronic Acid Based Materials for Biomedical Applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.-C. Recent Advances in Bio-Orthogonal and Dynamic Crosslinking of Biomimetic Hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Lee, H.-J.; Sen, A.; Bae, S.; Lee, J.S.; Webb, K. Poly(Ethylene Glycol) Diacrylate/Hyaluronic Acid Semi-Interpenetrating Network Compositions for 3-D Cell Spreading and Migration. Acta Biomater. 2015, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3D-Printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular Self-Healing Materials via Host-Guest Strategy between Cyclodextrin and Specific Types of Guest Molecules. Coord. Chem. Rev. 2021, 432, 213711. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic Hyaluronic Acid Hydrogel with Covalent Linked Gelatin as an Anti-Oxidative Bioink for Cartilage Tissue Engineering. Biofabrication 2022, 14, 014107. [Google Scholar] [CrossRef]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in Hydrogels Based on Dynamic Covalent Bonding and Prospects for Its Biomedical Application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Kodavaty, J.; Deshpande, A. Mechanical and Swelling Properties of Poly (Vinyl Alcohol) and Hyaluronic Acid Gels Used in Biomaterial Systems-a Comparative Study. Def. Sci. J. 2014, 64, 222–229. [Google Scholar] [CrossRef]
- Kodavaty, J. Poly (Vinyl Alcohol) and Hyaluronic Acid Hydrogels as Potential Biomaterial Systems-A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2022, 71, 103298. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Physical Properties of Crosslinked Hyaluronic Acid Hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef]
- Singh, D.; Tripathi, A.; Zo, S.; Singh, D.; Han, S.S. Synthesis of Composite Gelatin-Hyaluronic Acid-Alginate Porous Scaffold and Evaluation for in Vitro Stem Cell Growth and in Vivo Tissue Integration. Colloids Surf. B Biointerfaces 2014, 116, 502–509. [Google Scholar] [CrossRef]
- Cheaburu Yilmaz, C.; Pamfil, D.; Vasile, C.; Bibire, N.; Lupuşoru, R.-V.; Zamfir, C.-L.; Lupușoru, C. Toxicity, Biocompatibility, PH-Responsiveness and Methotrexate Release from PVA/Hyaluronic Acid Cryogels for Psoriasis Therapy. Polymers 2017, 9, 123. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically Crosslinked Silk-Hyaluronic Acid Hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, J.; Wang, L.; Zhang, D.; Zhang, J.; Guan, F.; Yao, M. Dual-Enzymatically Crosslinked Hyaluronic Acid Hydrogel as a Long-Time 3D Stem Cell Culture System. Biomed. Mater. 2020, 15, 045013. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, L.; Liu, S.; Tan, Y.; Xiao, J.; Cao, Y.; Chen, K.; Xiao, W.; Li, B.; Liao, X. Preparation of Silk Fibroin/Hyaluronic Acid Hydrogels with Enhanced Mechanical Performance by a Combination of Physical and Enzymatic Crosslinking. J. Biomater. Sci. Polym. Ed. 2021, 32, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, W.; Xiao, L.; Cao, Z.; Li, X.; Zhang, C.; Liao, L.; Liu, L. Enzymatically Cross-Linked Hyaluronic Acid/Graphene Oxide Nanocomposite Hydrogel with PH-Responsive Release. J. Biomater. Sci. Polym. Ed. 2015, 26, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Zhang, D.; Ma, S.; Zhang, J.; Gao, F.; Guan, F.; Yao, M. Dual-Enzymatically Crosslinked and Injectable Hyaluronic Acid Hydrogels for Potential Application in Tissue Engineering. RSC Adv. 2020, 10, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Wang, Z.-Y.; Ren, Z.-W.; Zhang, X.-W.; Wei, D.-X. Advances in Modified Hyaluronic Acid-Based Hydrogels for Skin Wound Healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef] [PubMed]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked Hyaluronic Acid Hydrogels: A Strategy to Functionalize and Pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and Quantitative Characterization of the Precursor Rheology of Hyaluronic Acid Hydrogels for Bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D Printable Hyaluronic Acid-Based Hydrogel for Its Potential Application as a Bioink in Tissue Engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef]
- Sepulveda, A.F.; Kumpgdee-Vollrath, M.; Franco, M.K.K.D.; Yokaichiya, F.; de Araujo, D.R. Supramolecular Structure Organization and Rheological Properties Modulate the Performance of Hyaluronic Acid-Loaded Thermosensitive Hydrogels as Drug-Delivery Systems. J. Colloid. Interface Sci. 2023, 630, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, K.; Katsumata, T.; Shimoboji, T.; Hashimoto, Y.; Kimura, T.; Akiyoshi, K.; Kishida, A. Variable Swelling Behavior of and Drug Encapsulation in a Maleimide-Modified Hyaluronic Acid Nanogel-Based Hydrogel. Polym. J. 2024, 56, 505–515. [Google Scholar] [CrossRef]
- Wan, T.; Fan, P.; Zhang, M.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Multiple Crosslinking Hyaluronic Acid Hydrogels with Improved Strength and 3D Printability. ACS Appl. Bio Mater. 2022, 5, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, J.; Zhang, K.; Fan, Y.; Zhang, X. Dynamic Mechanical and Swelling Properties of Maleated Hyaluronic Acid Hydrogels. Carbohydr. Polym. 2015, 123, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, X.; Yang, W.; Lin, C.; Tao, B.; Deng, Z.; Gao, P.; Yang, Y.; Cai, K. A PH-Responsive Hyaluronic Acid Hydrogel for Regulating the Inflammation and Remodeling of the ECM in Diabetic Wounds. J. Mater. Chem. B 2022, 10, 2875–2888. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in Vitro Drug Release of PH- and Temperature-Sensitive Double Cross-Linked Interpenetrating Polymer Network Hydrogels Based on Hyaluronic Acid/Poly (N-Isopropylacrylamide) for Transdermal Delivery of Luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Siu, W.S.; Kan, C.; Leung, P.-C.; Wanxue, C.; Chiou, J.-C. Influence of PH-Responsive Compounds Synthesized from Chitosan and Hyaluronic Acid on Dual-Responsive (PH/Temperature) Hydrogel Drug Delivery Systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Nikolajeva, V.; Loca, D. Effect of Crosslinking Strategy on the Biological, Antibacterial and Physicochemical Performance of Hyaluronic Acid and ε-Polylysine Based Hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A Chitosan-hyaluronic Acid Hydrogel Scaffold for Periodontal Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid–Based Injectable Hydrogels for Tissue Engineering Applications – Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-Linked Hyaluronic Acid Hydrogel Films: New Biomaterials for Drug Delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Varela-Aramburu, S.; Su, L.; Mosquera, J.; Morgese, G.; Schoenmakers, S.M.C.; Cardinaels, R.; Palmans, A.R.A.; Meijer, E.W. Introducing Hyaluronic Acid into Supramolecular Polymers and Hydrogels. Biomacromolecules 2021, 22, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.D.A.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the Elastic Modulus of Soft Culture Surfaces and Three-Dimensional Hydrogels Using Atomic Force Microscopy. Nat. Protoc. 2021, 16, 2418–2449. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Thanyacharoen, T.; Thongchai, K.; Techasakul, S.; Ummartyotin, S. Preparation of Chitosan/Hydrolyzed Collagen/Hyaluronic Acid Based Hydrogel Composite with Caffeic Acid Addition. Int. J. Biol. Macromol. 2020, 162, 1937–1943. [Google Scholar] [CrossRef]
- Vasi, A.-M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical Functionalization of Hyaluronic Acid for Drug Delivery Applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Benito Cid, S.; Pérez González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels 2022, 8, 223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, Q.; Fan, X. Preparation and Properties of a Chitosan–Hyaluronic Acid-Polypyrrole Conductive Hydrogel Catalyzed by Laccase. J. Polym. Environ. 2017, 25, 526–532. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Mahmood, A.; Munir, A.; Qalawlus, A.H.M.; Jabeen, N.; Kousar, M.; Anwar, Z. Hyaluronic Acid/Alginate-Based Biomimetic Hydrogel Membranes for Accelerated Diabetic Wound Repair. Int. J. Pharm. 2023, 643, 123244. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Van Belleghem, S.M.; Mahadik, B.; Snodderly, K.L.; Fisher, J.P. Overview of Tissue Engineering Concepts and Applications. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1289–1316. [Google Scholar]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef]
- Bagheri, S.; Bagher, Z.; Hassanzadeh, S.; Simorgh, S.; Kamrava, S.K.; Nooshabadi, V.T.; Shabani, R.; Jalessi, M.; Khanmohammadi, M. Control of Cellular Adhesiveness in Hyaluronic Acid-based Hydrogel through Varying Degrees of Phenol Moiety Cross-linking. J. Biomed. Mater. Res. A 2021, 109, 649–658. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Malekzadeh Kebria, M.; Khanmohammadi, M.; Bagher, Z. Development of Chitosan/Hyaluronic Acid Hydrogel Scaffolds via Enzymatic Reaction for Cartilage Tissue Engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Rajabnejad keleshteri, A.; Moztarzadeh, F.; Farokhi, M.; Mehrizi, A.A.; Basiri, H.; Mohseni, S.S. Preparation of Microfluidic-Based Pectin Microparticles Loaded Carbon Dots Conjugated with BMP-2 Embedded in Gelatin-Elastin-Hyaluronic Acid Hydrogel Scaffold for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.C.; Vu, P.; Modi, S.P.; Chung, P.E.; Landis, R.C.; Khaing, Z.Z.; Hardy, J.G.; Schmidt, C.E. Sacrificial Crystal Templated Hyaluronic Acid Hydrogels As Biomimetic 3D Tissue Scaffolds for Nerve Tissue Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1451–1459. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.J.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of Hyaluronic Acid in Tissue Engineering, Regenerative Medicine, and Nanomedicine: A Review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, D.; Yang, D.; Guo, Y.; Hu, R.; Li, Y.; Zan, X.; Zhang, X. Injectable, Self-Healing and Multiple Responsive Histamine Modified Hyaluronic Acid Hydrogels with Potentialities in Drug Delivery, Antibacterial and Tissue Engineering. Macromol. Rapid Commun. 2023, 44, 2200674. [Google Scholar] [CrossRef]
- Cheng, K.; Blusztajn, A.; Shen, D.; Li, T.-S.; Sun, B.; Galang, G.; Zarembinski, T.I.; Prestwich, G.D.; Marbán, E.; Smith, R.R.; et al. Functional Performance of Human Cardiosphere-Derived Cells Delivered in an in Situ Polymerizable Hyaluronan-Gelatin Hydrogel. Biomaterials 2012, 33, 5317–5324. [Google Scholar] [CrossRef]
- Prestwich, G.D. Evaluating Drug Efficacy and Toxicology in Three Dimensions: Using Synthetic Extracellular Matrices in Drug Discovery. Acc. Chem. Res. 2008, 41, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Ahmad, S.; Liu, Y.; Prestwich, G.D. Synthesis and Evaluation of Injectable, in Situ Crosslinkable Synthetic Extracellular Matrices for Tissue Engineering. J. Biomed. Mater. Res. A 2006, 79, 902–912. [Google Scholar] [CrossRef]
- Aleksander-Konert, E.; Paduszyński, P.; Zajdel, A.; Dzierżewicz, Z.; Wilczok, A. In Vitro Chondrogenesis of Wharton’s Jelly Mesenchymal Stem Cells in Hyaluronic Acid-Based Hydrogels. Cell Mol. Biol. Lett. 2016, 21, 11. [Google Scholar] [CrossRef]
- Kim, M.-H.; Nguyen, D.-T.; Kim, D.-D. Recent Studies on Modulating Hyaluronic Acid-Based Hydrogels for Controlled Drug Delivery. J. Pharm. Investig. 2022, 52, 397–413. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-Based Biomaterials for Pharmaceutical and Biomedical Applications: A Focus on Topical Drug Administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Yeruva, T.; Yang, S.; Doski, S.; Duncan, G.A. Hydrogels for Mucosal Drug Delivery. ACS Appl. Bio Mater. 2023, 6, 1684–1700. [Google Scholar] [CrossRef]
- Hou, X.; Zhong, D.; Chen, H.; Gu, Z.; Gong, Q.; Ma, X.; Zhang, H.; Zhu, H.; Luo, K. Recent Advances in Hyaluronic Acid-Based Nanomedicines: Preparation and Application in Cancer Therapy. Carbohydr. Polym. 2022, 292, 119662. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Hao, R.; Cui, Z.; Zhang, X.; Tian, M.; Zhang, L.; Rao, F.; Xue, J. Rational Design and Preparation of Functional Hydrogels for Skin Wound Healing. Front. Chem. 2022, 9, 839055. [Google Scholar] [CrossRef]
- Astaneh, M.E.; Fereydouni, N. A Focused Review on Hyaluronic Acid Contained Nanofiber Formulations for Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 253, 127607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Yang, Y.-P.; Jin, M.-Y.; Huang, S.; Zhuang, Z.-M.; Zhang, T.; Cao, L.-L.; Lin, X.-Y.; Chen, J.; et al. Versatile Dopamine-Functionalized Hyaluronic Acid-Recombinant Human Collagen Hydrogel Promoting Diabetic Wound Healing via Inflammation Control and Vascularization Tissue Regeneration. Bioact. Mater. 2024, 35, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable Multifunctional Hyaluronic Acid/Methylcellulose Hydrogels for Chronic Wounds Repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Cheng, H.-Y.; Ma, D.H.-K. Investigation of Overrun-Processed Porous Hyaluronic Acid Carriers in Corneal Endothelial Tissue Engineering. PLoS ONE 2015, 10, e0136067. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol Chitosan/Oxidized Hyaluronic Acid Hydrogel Film for Topical Ocular Delivery of Dexamethasone and Levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Guter, M.; Breunig, M. Hyaluronan as a Promising Excipient for Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 113, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the Natural History of Dry Eye Disease: A Prospective Registry-Based Cross-Sectional Study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Zhang, X.-W.; Mi, C.-H.; Qi, X.-Y.; Zhou, J.; Wei, D.-X. Recent Advances in Hyaluronic Acid-Based Hydrogels for 3D Bioprinting in Tissue Engineering Applications. Smart Mater. Med. 2023, 4, 59–68. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, e2000095. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-Inspired Hydrogel Composed of Hyaluronic Acid and Alginate as a Potential Bioink for 3D Bioprinting of Articular Cartilage Engineering Constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Suo, A.; Xu, W.; Wang, Y.; Sun, T.; Ji, L.; Qian, J. Dual-Degradable and Injectable Hyaluronic Acid Hydrogel Mimicking Extracellular Matrix for 3D Culture of Breast Cancer MCF-7 Cells. Carbohydr. Polym. 2019, 211, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Liang, J.; Bierman, R.D.; Sohrabi, A.; Karam, J.; Holley, S.M.; Cepeda, C.; Walthers, C.M. Peptide-modified, Hyaluronic Acid-based Hydrogels as a 3D Culture Platform for Neural Stem/Progenitor Cell Engineering. J. Biomed. Mater. Res. A 2019, 107, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.J.; Constantinou, P.E.; Sablatura, L.K.; Doty, N.J.; Carson, D.D.; Farach-Carson, M.C.; Harrington, D.A.; Zarembinski, T.I. Multilayered, Hyaluronic Acid-Based Hydrogel Formulations Suitable for Automated 3D High Throughput Drug Screening of Cancer-Stromal Cell Cocultures. Adv. Healthc. Mater. 2015, 4, 1664–1674. [Google Scholar] [CrossRef]
- Gurski, L.A.; Jha, A.K.; Zhang, C.; Jia, X.; Farach-Carson, M.C. Hyaluronic Acid-Based Hydrogels as 3D Matrices for in Vitro Evaluation of Chemotherapeutic Drugs Using Poorly Adherent Prostate Cancer Cells. Biomaterials 2009, 30, 6076–6085. [Google Scholar] [CrossRef]




















| Cross-Linking Method | Reagents/Conditions Used | Applications | References |
|---|---|---|---|
| Carbodiimide cross-linking | EDC | Tissue engineering, drug delivery | [22] |
| Diisocyanate cross-linking | HDI, bis (β- isocyanatoethyl) disulp hide | Tissue engineering, wound healing, drug delivery | [23] |
| Michael addition | Thiol groups (cysteine, DTT), -VS, -MAL, -AC | Tissue engineering, drug delivery, controlled drug/gene release | [24,25,26,27,28] |
| Esterification | EDC/HOBt | Drug delivery, wound healing, tissue engineering | [29,30] |
| Diels–Alder reaction | Norbornene, tetrazine, furan, maleimide | Injectable hydrogels, photo-degradable hydrogels, controlled drug release | [31,32] |
| Photo cross-linking | Photo-initiator, UV, or visible light | Tissue engineering, wound healing, controlled drug release | [33,34] |
| Thiol-ene click | (Meth)acrylate and thiol functional groups without initiators under physiological conditions | cell culture, contact lenses | [35,36] |
| Ether reaction | BDDE; DVS under room temperature conditions | Drug delivery | [36] |
| Amidation | EDC, CMPI, CDMT | Drug delivery | [35,36] |
| Hydrazone linkage | Hyaluronic acid Adipic acid dihydrazide (HA-ADH) reacted with aldehydes or ketones | Drug delivery | [37,38,39] |
| Temperature-induced gelation | Thermo-responsive polymers (PNIPAAm) | Injectable hydrogels, tissue engineering | [40,41] |
| Covalent augmentation | PEGDA | Enhanced mechanical properties, controlled drug delivery | [42] |
| Freeze–thawing | Repeated freezing and thawing | Porous structure, controlled drug release | [43,44] |
| Enzymatic cross-linking | Horseradish peroxidase, tyramine | Tissue engineering, drug delivery, wound healing | [45,46] |
| Ophthalmology Application | Target | HA Function |
|---|---|---|
| Artificial tear and eye drops | Ocular surface | 1. Increase the moisture retention [99] 2. Better tear film stability, ocular surface regularity, and quantity of conjunctival goblet cells [166] |
| Tissue engineering | Corneal | Benefit of cell growth and wound healing [163] |
| In situ gel | Ocular surface | 1. Help the drug absorption and drug delivery [47] 2. Adjust the viscosity and degradation time [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholamali, I.; Vu, T.T.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials 2024, 17, 2439. https://doi.org/10.3390/ma17102439
Gholamali I, Vu TT, Jo S-H, Park S-H, Lim KT. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials. 2024; 17(10):2439. https://doi.org/10.3390/ma17102439
Chicago/Turabian StyleGholamali, Iman, Trung Thang Vu, Sung-Han Jo, Sang-Hyug Park, and Kwon Taek Lim. 2024. "Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications" Materials 17, no. 10: 2439. https://doi.org/10.3390/ma17102439





