Study of Oxygen Reduction Reaction on Polycrystalline Rhodium in Acidic and Alkaline Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterization of Rh(poly) in Acid and Alkaline Media
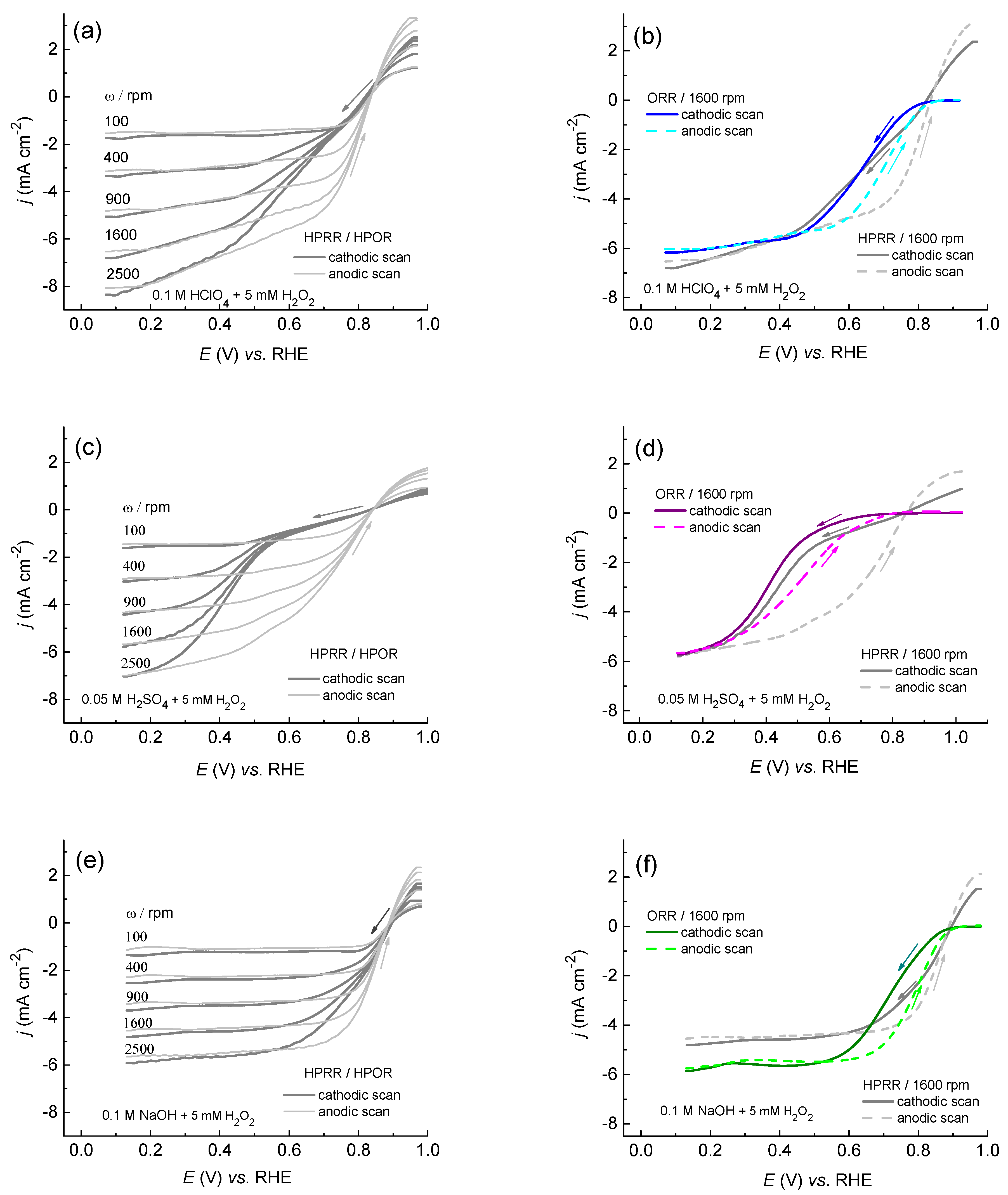
2.2. Oxygen Reduction on Rh(poly) in Acidic and Alkaline Media
2.2.1. ORR on Rh(poly) in Acidic Media
2.2.2. ORR on Rh(poly) in Alkaline Media
2.2.3. Hydrogen Peroxide Oxidation/Reduction on Rh(poly) in Acid and Alkaline Media
2.2.4. Stability of Rh(poly) during ORR in Acidic and Alkaline Media
2.3. Comparison of the Catalytic Activity of Rh(poly) in Acidic and Alkaline Media
2.4. Comparison of the ORR Activity of Rh(poly) with Rh-Based Catalysts
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for the oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden-Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting electrocatalytic activity of single atom catalysts supported on nitrogen-doped carbon through N coordination environment engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Li, L.; Shang, Y.; Zhu, Q.; Han, M.; Tu, X. Promises of engineering Rh-based nanostructures for advanced electrocatalysis. Int. J. Hydrogen Energy 2024, 51, 1313–1336. [Google Scholar] [CrossRef]
- Bai, J.; Han, S.-H.; Peng, R.-L.; Zeng, J.-H.; Jiang, J.X.; Chen, Y. Ultrathin rhodium oxide nanosheet nanoassemblies: Synthesis, morphological stability, and electrocatalytic application. ACS Appl. Mater. Interfaces 2017, 9, 17195–17200. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-Q.; Xue, Q.; Zhao, Y.; Li, X.-F.; Jin, P.-J.; Chen, Y. Selective etching induced synthesis of hollow Rh nanospheres electrocatalyst for alcohol oxidation reactions. Small 2018, 14, 1801239. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xing, S.-H.; Zhu, Y.-Y.; Jiang, J.-X.; Zeng, J.-H.; Chen, Y. Polyallylamine-Rh nanosheet nanoassemblies−carbon nanotubes organic-inorganic nanohybrids: A electrocatalyst superior to Pt for the hydrogen evolution reaction. J. Power Sources 2018, 385, 32–38. [Google Scholar] [CrossRef]
- Akbayrak, M.; Önal, A.M. High durability and electrocatalytic activity toward hydrogen evolution reaction with ultralow rhodium loading on titania. J. Electrochem. Soc. 2020, 167, 156501. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Das, S.; Adhikary, K.; Ghangrekar, M.M.; Mitra, A. Using rhodium as a cathode catalyst for enhancing performance of microbial fuel cell. Int. J. Hydrogen Energy 2019, 44, 22218–22222. [Google Scholar] [CrossRef]
- Park, S.; Park, N.; Nallal, M.; Yusuf, M.; Park, S.; Lee, J.-M.; Park, K.H. Facile synthesis of nitrogen-doped carbon-supported rhodium–cobalt alloy electrocatalyst for oxygen reduction reaction. Processes 2022, 10, 2357. [Google Scholar] [CrossRef]
- Zelenay, P.; Horányi, G.; Rhee, C.K.; Wieckowski, A. Voltammetric and radioactive labeling studies of single crystal and polycrystalline rhodium electrodes in sulfate-containing electrolytes. J. Electroanal. Chem. 1991, 300, 499–519. [Google Scholar] [CrossRef]
- Rhee, C.K.; Wasberg, M.; Zelenay, P.; Wieckowski, A. Reduction of perchlorate on rhodium and its specificity to surface crystallographic orientation. Catal. Lett. 1991, 10, 149–164. [Google Scholar] [CrossRef]
- Xu, Q.; Linke, U.; Bujak, R.; Wandlowski, T. Preparation and electrochemical characterization of low-index rhodium single crystal electrodes in sulfuric acid. Electrochim. Acta 2009, 54, 5509–5521. [Google Scholar] [CrossRef]
- Štrbac, S.; Smiljanić, M.; Rakočević, Z. Spontaneously deposited Rh on Au(111) observed by AFM and XPS: Electrocatalysis of hydrogen evolution. J. Electrochem. Soc. 2016, 163, D3027–D3033. [Google Scholar] [CrossRef]
- Potgieter, M.; Parrondo, J.; Ramani, V.K.; Kriek, R.J. Evaluation of polycrystalline platinum and rhodium surfaces for the electro-oxidation of aqueous sulfur dioxide. Electrocatalysis 2016, 7, 50–59. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Jurczakowski, R.; Lasia, A. Kinetics of hydrogen underpotential deposition at polycrystalline rhodium in acidic solutions. Electrochim. Acta 2011, 56, 5746–5753. [Google Scholar] [CrossRef]
- Jakšić, M.M.; Johansen, B.; Tunold, R. Electrochemical behavior of rhodium in alkaline and acidic solutions of heavy and regular water. Int. J. Hydrogen Energy 1994, 19, 35–51. [Google Scholar] [CrossRef]
- Karschin, A.; Katsounaros, I.; Klemm, S.O.; Meier, J.C.; Mayrhofer, K.J.J. Degradation of polycrystalline rhodium and rhodium nanoparticles. Electrochim. Acta 2012, 70, 355–359. [Google Scholar] [CrossRef]
- Martinović, J.; Šepa, D.; Vojnović, M.V.; Damjanović, A. Kinetics of electrochemical reduction of oxygen at rhodium. Electrochim. Acta 1988, 33, 1267–1272. [Google Scholar] [CrossRef]
- Šepa, D.; Vojnović, M.V.; Damjanović, A. Reaction intermediates as a controlling factor in the kinetics and mechanism of oxygen reduction at platinum electrodes. Electrochim. Acta 1981, 26, 781–793. [Google Scholar] [CrossRef]
- Guan, J.; Wen, X.; Zhang, Q.; Duan, Z. Atomic rhodium catalysts for hydrogen evolution and oxygen reduction reactions. Carbon 2020, 164, 121–128. [Google Scholar] [CrossRef]
- Hwang, G.S.; Shin, W.; Yim, G.; Choi, J.H.; Kim, Y.-K.; Jang, H.; Kim, Y.-R. Morphology- and composition-controlled silver-containing rhodium nanoparticles for the oxygen reduction reaction. Bull. Korean Chem. Soc. 2022, 43, 1240–1246. [Google Scholar] [CrossRef]
- Ziegelbauer, J.M.; Gatewood, D.; Gullá, A.F.; Guinel, M.J.-F.; Ernst, F.; Ramaker, D.E.; Mukherjee, S. Fundamental investigation of oxygen reduction reaction on rhodium sulfide-based chalcogenides. J. Phys. Chem. C 2009, 113, 6955–6968. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Siwek, H.; Czerwinski, A. Electrochemical behavior of thin polycrystalline rhodium layers studied by cyclic voltammetry and quartz crystal microbalance. Electrochim. Acta 2007, 52, 4560–4565. [Google Scholar] [CrossRef]
- Woods, R. Hydrogen adsorption on platinum, iridium, and rhodium electrodes at reduced temperatures and the determination of real surface area. J. Electroanal. Chem. Interf. Electrochem. 1974, 49, 217–226. [Google Scholar] [CrossRef]
- Rand, D.A.J.; Woods, R. The nature of adsorbed oxygen on rhodium, palladium and gold electrodes. J. Electroanal. Chem. Interf. Electrochem. 1971, 31, 29–38. [Google Scholar] [CrossRef]
- Stonehart, P.; Kozlowska, H.A.; Conway, B.E. Potentiodynamic examination of electrode kinetics for electroactive adsorbed species: Applications to the reduction of noble metal surface oxides. Proc. Boy. Soc. A 1969, 310, 541–563. [Google Scholar]
- Oliveira, R.T.S.; Santos, M.C.; Bulhões, L.O.S.; Pereira, E.C. Rh electrodeposition on Pt in acidic medium: A study using cyclic voltammetry and an electrochemical quartz crystal microbalance. J. Electroanal. Chem. 2004, 569, 233–240. [Google Scholar] [CrossRef]
- Peuckert, M. A comparison of thermally and electrochemically prepared oxidation adlayers on rhodium: Chemical nature and thermal stability. Surf. Sci. 1984, 141, 500–514. [Google Scholar] [CrossRef]
- Villard, F.; Jerkiewicz, G. Comprehensive studies of formation and reduction of surface oxides at rhodium electrodes at 298 K. Can. J. Chem. 1997, 75, 1656–1665. [Google Scholar] [CrossRef]
- Pallotta, C.; de Tacconi, N.R.; Arvia, A.J. Potentiodynamic behavior of the rhodium/H2SO4(aq) interface in the potential range of the hydrogen and oxygen electrosorption. Electrochim. Acta 1981, 26, 261–273. [Google Scholar]
- Florit, M.I.; Bolzán, A.E.; Arvia, A.J. Reactions involving H, OH and O species on rhodium in H2SO4·12H2O and HClO4·5.5H2O in the range 198–298 K. J. Electroanal. Chem. 1995, 394, 253–262. [Google Scholar] [CrossRef]
- Wasberg, M.; Horányi, G. The reversibility and overlapping of hydrogen and oxygen adsorption in the “double-layer” region of rhodized electrodes in H2SO4 solutions (the decomposition of water molecules on rhodium surfaces). J. Electroanal. Chem. 1995, 386, 213–219. [Google Scholar] [CrossRef]
- Vuković, M. Electrochemical investigation of an electrodeposited rhodium electrode in acid solutions. J. Electroanal. Chem. 1988, 242, 97–105. [Google Scholar] [CrossRef]
- Štrbac, S. The effect of pH on oxygen and hydrogen peroxide reduction on polycrystalline Pt electrode. Electrochim. Acta 2011, 56, 1597–1604. [Google Scholar] [CrossRef]
- Parajon Costa, B.; Giordano, M.C.; Pallotta, C.D.; Arvia, A.J. Voltammetry of polycrystalline rhodium in 1 M H2SO4 at different temperatures in the 0–65°C range. J. Electroanal. Chem. 1986, 199, 381–394. [Google Scholar] [CrossRef]
- Rakotondrainibe, A.; Beden, B.; Lamy, C. Investigation of the early stages of Hads and OHads adsorption on rhodium in alkaline medium Part I: Approaches from graphical treatments of cyclic voltammograms based on a langmuirian isotherm. J. Electroanal. Chem. 1994, 379, 455–465. [Google Scholar] [CrossRef]
- Cataldi, Z.; Lezna, R.O.; Giordano, M.C.; Arvia, A.J. Voltammetric study of polycrystalline rhodium in alkaline solutions at different temperatures. J. Electroanal. Chem. Interf. Electrochem. 1989, 261, 61–75. [Google Scholar] [CrossRef]
- Hoare, J.P. Oxygen. In Standard Potentials in Aqueous Solutions; Bard, A.J., Parsons, R., Jordan, J., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 49–66. [Google Scholar]
- Wakabayashi, N.; Takeuchi, M.; Itagaki, M.; Uchida, H.; Watanabe, M. Temperature-dependence of oxygen reduction activity at a platinum electrode in an acidic electrolyte solution investigated with a channel flow double electrode. J. Electroanal. Chem. 2005, 574, 339–346. [Google Scholar] [CrossRef]
- Markovic, N.M.; Adzic, R.R.; Cahan, B.D.; Yeager, E.B. Structural effects in electrocatalysis: Oxygen reduction on platinum low index single-crystal surfaces in perchloric acid solutions. J. Electroanal. Chem. 1994, 377, 249–259. [Google Scholar] [CrossRef]
- Srejić, I.; Rakočević, Z.; Nenadović, M.; Štrbac, S. Oxygen reduction on polycrystalline palladium in acid and alkaline solutions: Topographical and chemical Pd surface changes. Electrochim. Acta 2015, 169, 22–31. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Feliu, J.M.; Ticianelli, E. Oxygen reduction on platinum surfaces in acid media: Experimental evidence of a CECE/DISP initial reaction path. ACS Catal. 2019, 9, 2238–2251. [Google Scholar] [CrossRef]
- Štrbac, S.; Adžić, R. Oxygen reduction on single crystal gold electrodes in acid solutions. J. Serb. Chem. Soc. 1992, 57, 835–848. [Google Scholar]
- Markovic, N.; Gasteiger, H.; Ross, P.N. Kinetics of oxygen reduction on Pt(hkl) electrodes: Implications for the crystallite size effect with supported Pt electrocatalysts. J. Electrochem. Soc. 1997, 144, 1591. [Google Scholar] [CrossRef]
- Li, M.F.; Liao, L.W.; Yuan, D.F.; Mei, D.; Chen, Y.-X. pH effect on oxygen reduction reaction at Pt(111) electrode. Electrochim. Acta 2013, 110, 780–789. [Google Scholar] [CrossRef]
- Golubović, J.; Rakočević, L.; Vasiljević Radović, D.; Štrbac, S. Improved oxygen reduction on GC-supported large-sized Pt nanoparticles by the addition of Pd. Catalysts 2022, 12, 968. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Norskov, J.K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, S.; Adžić, R.R. The influence of OH- chemisorption on the catalytic properties of gold single crystal surfaces for oxygen reduction in alkaline solutions. J. Electroanal. Chem. 1996, 403, 169–181. [Google Scholar] [CrossRef]
- Pan, S.; Yu, X.; Long, X.; Chang, C.; Yang, Z. Ultrafine rhodium selenides enable efficient oxygen reduction reaction catalysis. Sustain. Energy Fuels. 2021, 5, 6197–6201. [Google Scholar] [CrossRef]
- Sookhakian, M.; Tong, G.B.; Alias, Y. In-situ electrodeposition of rhodium nanoparticles anchored on reduced graphene oxide nanosheets as an efficient oxygen reduction electrocatalyst. Appl. Organometal. Chem. 2020, 34, e5370. [Google Scholar] [CrossRef]
- Ahn, M.; Cha, I.Y.; Cho, J.; Ham, H.C.; Sung, Y.-E.; Yoo, S.J. Rhodium-tin binary nanoparticle—A strategy to develop an alternative electrocatalyst for oxygen reduction. ACS Catal. 2017, 7, 5796–5801. [Google Scholar] [CrossRef]








| Solution | Eonset (V) | E1/2 (V) | Tafel Slope (mV dec−1) at Low η | Tafel Slope (mV dec−1) at High η |
|---|---|---|---|---|
| 0.1 M HClO4 | 0.85 | 0.64 | 60 | 120 |
| 0.05 M H2SO4 | 0.74 | 0.42 | 80 | 200 |
| 0.1 M NaOH | 0.90 | 0.72 | 60 | 120 |
| Solution | Eonset (V) | E1/2 (V) | Tafel Slope (mV dec−1) at Low η | Tafel Slope (mV dec−1) at High η |
|---|---|---|---|---|
| 0.1 M HClO4 | 0.87 | 0.70 | 60 | 120 |
| 0.05 M H2SO4 | 0.82 | 0.51 | 70 | 180 |
| 0.1 M NaOH | 0.92 | 0.78 | 60 | 120 |
| Catalyst | Solution | Eonse (V) | E1/2 (V) | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|---|---|
| Rh@NG | 0.1 M HClO4 | 0.85 | 0.740 | - | [22] |
| Rh(poly) | 0.1 M HClO4 | 0.85 | 0.64 | 60 | This work |
| Rh/C (5 wt%) | 0.5 M H2SO4 | 0.75 | - | 119 | [23] |
| sRhNPs | 0.5 M H2SO4 | 0.79 | - | 68.3 | [23] |
| fRhNPs | 0.5 M H2SO4 | 0.70 | - | 89.5 | [23] |
| pRhNPs | 0.5 M H2SO4 | 0.70 | - | 82.2 | [23] |
| Rh(poly) | 0.05 M H2SO4 | 0.74 | 0.42 | 80 | This work |
| Rh@G | 0.1 M KOH | - | 0.737 | - | [22] |
| Rh@NG | 0.1 M KOH | - | 0.848 | - | [22] |
| Rh/C | 1 M KOH | 1.02 | 0.833 | 70 | [51] |
| Rh3Se4/C | 1 M KOH | 1.10 | 0.840 | 71 | [51] |
| Rh@rGO/GCE | 0.1 M KOH | 0.87 | - | - | [52] |
| Rh/C | 0.1 M KOH | 0.85 | 0.72 | 60 | [53] |
| Rh3Sn1/C | 0.1 M KOH | 0.90 | 0.84 | 43 | [53] |
| Rh(poly) | 0.1 M NaOH | 0.90 | 0.72 | 60 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubović, J.; Varničić, M.; Štrbac, S. Study of Oxygen Reduction Reaction on Polycrystalline Rhodium in Acidic and Alkaline Media. Catalysts 2024, 14, 327. https://doi.org/10.3390/catal14050327
Golubović J, Varničić M, Štrbac S. Study of Oxygen Reduction Reaction on Polycrystalline Rhodium in Acidic and Alkaline Media. Catalysts. 2024; 14(5):327. https://doi.org/10.3390/catal14050327
Chicago/Turabian StyleGolubović, Jelena, Miroslava Varničić, and Svetlana Štrbac. 2024. "Study of Oxygen Reduction Reaction on Polycrystalline Rhodium in Acidic and Alkaline Media" Catalysts 14, no. 5: 327. https://doi.org/10.3390/catal14050327






