An Effective Method for the Evaluation of the Enantiomeric Purity of 1,2-Diacyl-sn-glycero-3-phosphocholine-Based Lipids by NMR Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis of (R)-(2-(((1-Phenylethyl)amino)methyl)phenyl)boronic Acid ((R)-CDA) [20,21]
2.3. Procedure for Phospholipid Methanolysis
2.4. Procedure for GPC-Chiral Boronic Acid Reaction
2.5. Procedure for Analysis of GPC-Chiral Boronic Acid Adduct by 1H NMR
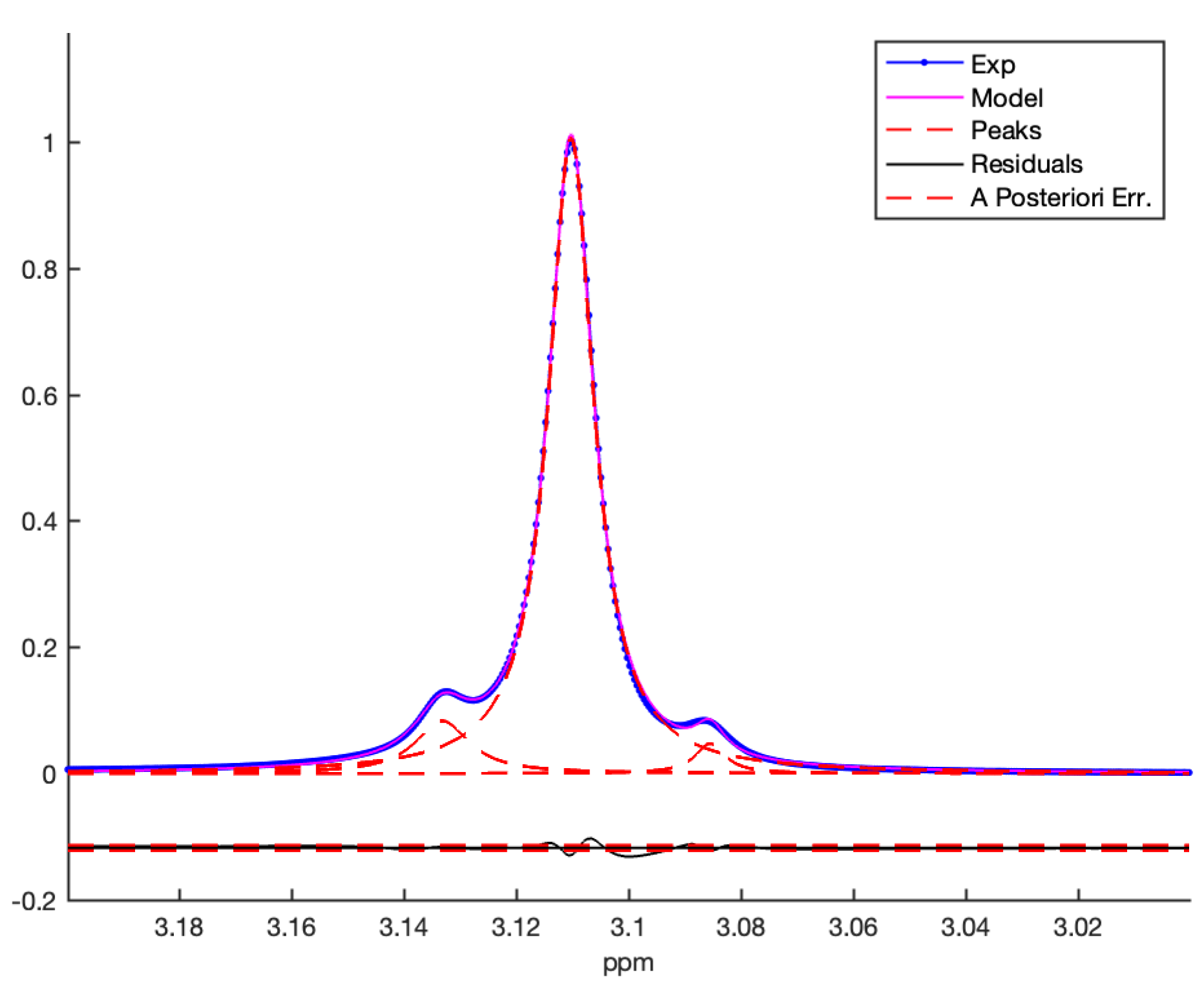
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Chand, S. Recent progress on phospholipases: Different sources, in: Assay Methods, Industrial Potential and Pathogenicity. Appl. Biochem. Biotechnol. 2011, 164, 991–1022. [Google Scholar] [CrossRef]
- Álvarez-Benedicto, E.; Farbiak, L.; Márquez Ramírez, M.; Wang, X.; Johnson, L.T.; Mian, O.; Guerrero, E.D.; Siegwart, D.J. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater. Sci. 2022, 10, 549. [Google Scholar] [CrossRef]
- Martin, H.S.; Podolsky, K.A.; Devaraj, N.K. Probing the Role of Chirality in Phospholipid Membranes. ChemBioChem 2021, 22, 3148–3157. [Google Scholar] [CrossRef]
- Wachtershauser, G. From pre-cells to Eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Yamagishi, A. Stability of Heterochiral Hybrid Membrane Made of Bacterial sn-G3P Lipids and Archaeal sn-G1P Lipids. Biochemistry 2011, 50, 4114–4120. [Google Scholar] [CrossRef]
- Caforio, A.; Siliakus, M.F.; Exterkate, M.; Jain, S.; Jumde, V.R.; Andringa, R.L.H.; Kengen, S.W.M.; Minnaard, A.J.; Driessen, A.J.M.; van der Oost, J. Converting Escherichia coli into an archaebacterium with a hybrid heterochiral membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3704–3709. [Google Scholar] [CrossRef]
- Altamura, E.; Comte, A.; D’Onofrio, A.; Roussillon, C.; Fayolle, D.; Buchet, R.; Mavelli, F.; Stano, P.; Fiore, M.; Strazewski, P. Racemic Phospholipids for Origin of Life Studies. Symmetry 2020, 12, 1108. [Google Scholar] [CrossRef]
- Fiore, M.; Buchet, R. Symmetry Breaking of Phospholipids. Symmetry 2020, 12, 1488. [Google Scholar] [CrossRef]
- Hanashima, S.; Yano, Y.; Murata, M. Enantiomers of phospholipids and cholesterol: A key to decipher lipid-lipid interplay in membrane. Chirality 2020, 32, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Suga, K.; Umakoshi, H. Chiral Recognition of L-Amino Acids on Liposomes Prepared with l-Phospholipid. ACS Appl. Mater. Interfaces 2015, 7, 21065–21072. [Google Scholar] [CrossRef]
- Semproli, R.; Robescu, M.S.; Cambò, M.; Mema, K.; Bavaro, T.; Rabuffetti, M.; Ubiali, D.; Speranza, G. Chemical and Enzymatic Approaches to Esters of sn-Glycero-3-Phosphoric Acid. Eur. J. Org. Chem. 2021, 2021, 4027–4037. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Trauner, D. Concise Synthesis of Glycerophospholipids. J. Org. Chem. 2023, 88, 11253–11257. [Google Scholar] [CrossRef]
- De Ferra, L.; Massa, A.; Di Mola, A.; Diehl, B. An effective method for the determination of the enantio-purity of L-α-glycerophosphocholine (L-α-GPC). J. Pharm. Biomed. Anal. 2020, 183, 113152. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, Y. Chiral separation of glycerolipids by high-performance liquid chromatography. J. Lipid Nutr. 2012, 21, 27–34. [Google Scholar] [CrossRef]
- Taniguchi, T.; Manai, D.; Shibata, M.; Itabashi, Y.; Monde, K. Stereochemical Analysis of Glycerophospholipids by Vibrational Circular Dichroism. J. Am. Chem. Soc. 2015, 137, 12191–12194. [Google Scholar] [CrossRef]
- Tomassoni, D.; Catalani, A.; Cinque, C.; Di Tullio, M.A.; Tayebati, S.K.; Cadoni, A.; Nwankwo, I.E.; Traini, E.; Amenta, F. Effects of cholinergic enhancing drugs on cholinergic transporters in the brain and peripheral blood lymphocytes of spontaneously hypertensive rats. Curr. Alzheimer Res. 2012, 9, 120–127. [Google Scholar] [CrossRef]
- Carlsson, A. Brain Neurotransmitters in Aging and Dementia: Similar Changes Across Diagnostic Dementia. Groups Gerontol. 1987, 33, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.H.; Regan, C.J.; Anslyn, E.V. A General Protocol for Creating High-throughput Screening Assays for Reaction Yield and Enantiomeric Excess Applied to Hydrobenzoin. Proc. Natl. Acad. Sci. USA 2009, 106, 10487–10492. [Google Scholar] [CrossRef]
- Zhu, L.; Anslyn, E.V. Facile Quantification of Enantiomeric Excessand Concentration with Indicator-Displacement Assays: An Example in the Analyses of a-Hydroxyacids. J. Am. Chem. Soc. 2004, 126, 3676–3677. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Summa, F.F.; Oliva, P.; Lelj, F.; Remiddi, S.; Silvani, L.; Massa, A. Synergistic Properties of Arabinogalactan (AG) and Hyaluronic Acid (HA) Sodium Salt Mixtures. Molecules 2021, 26, 7246. [Google Scholar] [CrossRef] [PubMed]
- De Ferra, L.; Anibaldi, M.; Zenoni, M. Process for the Purification of L-Alpha-Glycerophosphorylcholine. PCT WO 2015/189766 A1, 17 December 2015. [Google Scholar]
- Tronconi, G. Process for the Preparation of L-Alpha-Glycerophosphorylcholine and L-Alpha-Glycerophosphorylethanoamine. WO 90/13552, 15 November 1990. [Google Scholar]
- Monaco, G.; Aquino, F.; Zanasi, R.; Herrebout, W.; Bultinck, P.; Massa, A. Model-Averaging of Ab Initio Spectra for the Absolute Configuration Assignment via Vibrational Circular Dichroism. Phys. Chem. Chem. Phys. 2017, 19, 28028–28036. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, A.; de Ferra, L.; Anibaldi, M.; Monaco, G.; Massa, A. An Effective Method for the Evaluation of the Enantiomeric Purity of 1,2-Diacyl-sn-glycero-3-phosphocholine-Based Lipids by NMR Analysis. Symmetry 2024, 16, 624. https://doi.org/10.3390/sym16050624
Di Mola A, de Ferra L, Anibaldi M, Monaco G, Massa A. An Effective Method for the Evaluation of the Enantiomeric Purity of 1,2-Diacyl-sn-glycero-3-phosphocholine-Based Lipids by NMR Analysis. Symmetry. 2024; 16(5):624. https://doi.org/10.3390/sym16050624
Chicago/Turabian StyleDi Mola, Antonia, Lorenzo de Ferra, Mauro Anibaldi, Guglielmo Monaco, and Antonio Massa. 2024. "An Effective Method for the Evaluation of the Enantiomeric Purity of 1,2-Diacyl-sn-glycero-3-phosphocholine-Based Lipids by NMR Analysis" Symmetry 16, no. 5: 624. https://doi.org/10.3390/sym16050624





