Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications
Abstract
:1. Introduction
- Sweat collection module: Sweat collection modules serve as a pivotal component in wearable healthcare devices, enabling the non-invasive monitoring of physiological markers [26,27]. These modules employ skin patches and microfluidic chips to gather perspiration, which contains vital biochemical indicators of health [28]. The design of sweat collection modules hinges on material selection, microfluidic architecture, and preservation strategies to maintain sample integrity [29]. Advances in material science have led to the use of novel adsorbents and biocompatible substrates, enhancing both comfort and functionality [25]. Concurrently, innovations in microfluidics have improved fluidic control and analytical precision, expanding the scope of applications [30].
- Sweat detection module: Sweat detection modules are integral for quantifying biomarkers in perspiration, utilizing a variety of sensors including biosensing electrodes, nanosensors, and more [31]. These modules consist of a flexible substrate and detection electrodes, with different sensors tailored to detect specific biological molecules or markers [32,33]. Enzymatic sensors rely on enzyme–analyte reactions to generate electrical signals, exemplified by glucose sensors that employ glucose oxidase [34]. Ion-selective electrodes (ISEs) detect particular ions using ionophores with high affinity, such as chloride ISEs for cystic fibrosis diagnostics [35]. Antibody-based sensors bind target molecules, triggering measurable responses, useful for identifying proteins or biomarkers [36]. Nanomaterial-based sensors leverage carbon nanotubes, graphene [37], or metal nanoparticles to amplify sensitivity and selectivity, capitalizing on their unique characteristics and large surface area for efficient analyte detection in sweat [38]. Electrode materials vary depending on the biomarkers under investigation, yet regardless of the detection approach or substance analyzed, the underlying flexible substrate serves as a common platform for different sensing techniques [39,40].
- Data-processing module: The signal-processing module serves as a critical component in the translation and analysis of sensor data, bridging the gap between raw sensor outputs and meaningful biological insights [41]. It commences by transducing signals from physical to electrical formats, potentially employing amplification and filtering techniques to enhance signal quality [42]. Subsequently, the module applies sophisticated analytical methods, including pattern recognition and data-processing algorithms, to extract and elucidate significant biological signatures [43,44]. Furthermore, it facilitates the conveyance of processed data to various external devices and cloud services via a combination of wired interfaces (e.g., USB interface or serial port) and wireless protocols (e.g., Bluetooth, Wi-Fi, RFID, etc.) [45]. Generally, the design of the module integrates proficiency in both electronic hardware and software algorithms, ensuring efficient and reliable data management, which implies that progress in materials science has exerted less influence on the development of this segment [46].
- Self-powering module: The self-powering module offers a sustainable and convenient solution for powering sensors. Unlike traditional power supply units, such as batteries or external adapters, this module harnesses environmental energy, aligning with green energy principles [47]. It is particularly suitable for wearable electrochemical sweat sensors, as it is lightweight, compact, and provides a stable power source without posing any harm to the human body [48]. The self-powering module encompasses various technologies, including solar cells, triboelectric nanogenerators (TENGs), and biofluid cells (BFCs) [49]. These features make it an innovative and efficient choice for sensor applications, promoting both practicality and environmental consciousness. Each of these self-supplied electric methods has its advantages and disadvantages, and it is necessary to choose different technologies for different scenarios, and then choose different innovative materials [50].

2. Wearable Non-Invasive Electrochemical Sweat Sensors
2.1. Innovative Materials for Sweat Collection
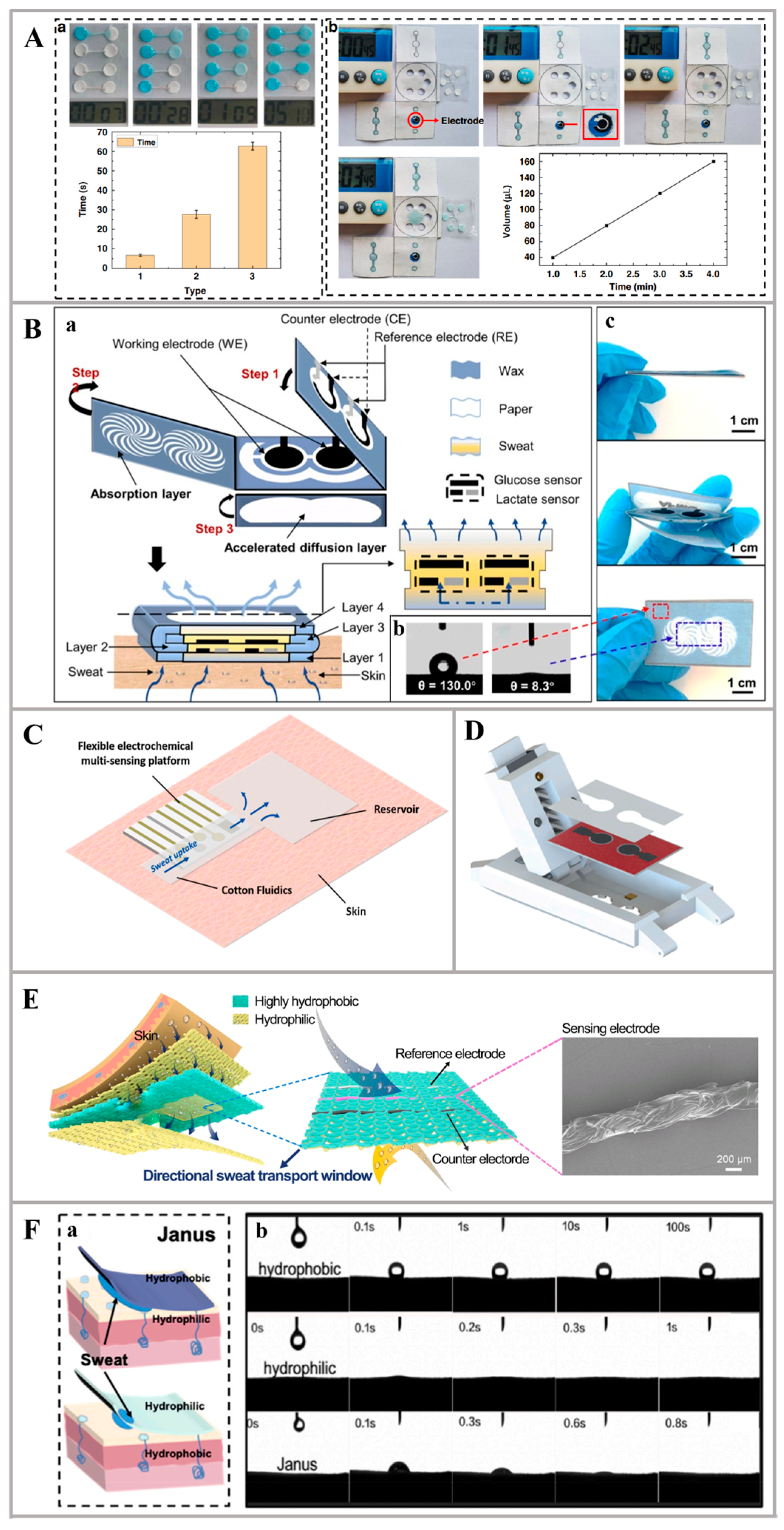
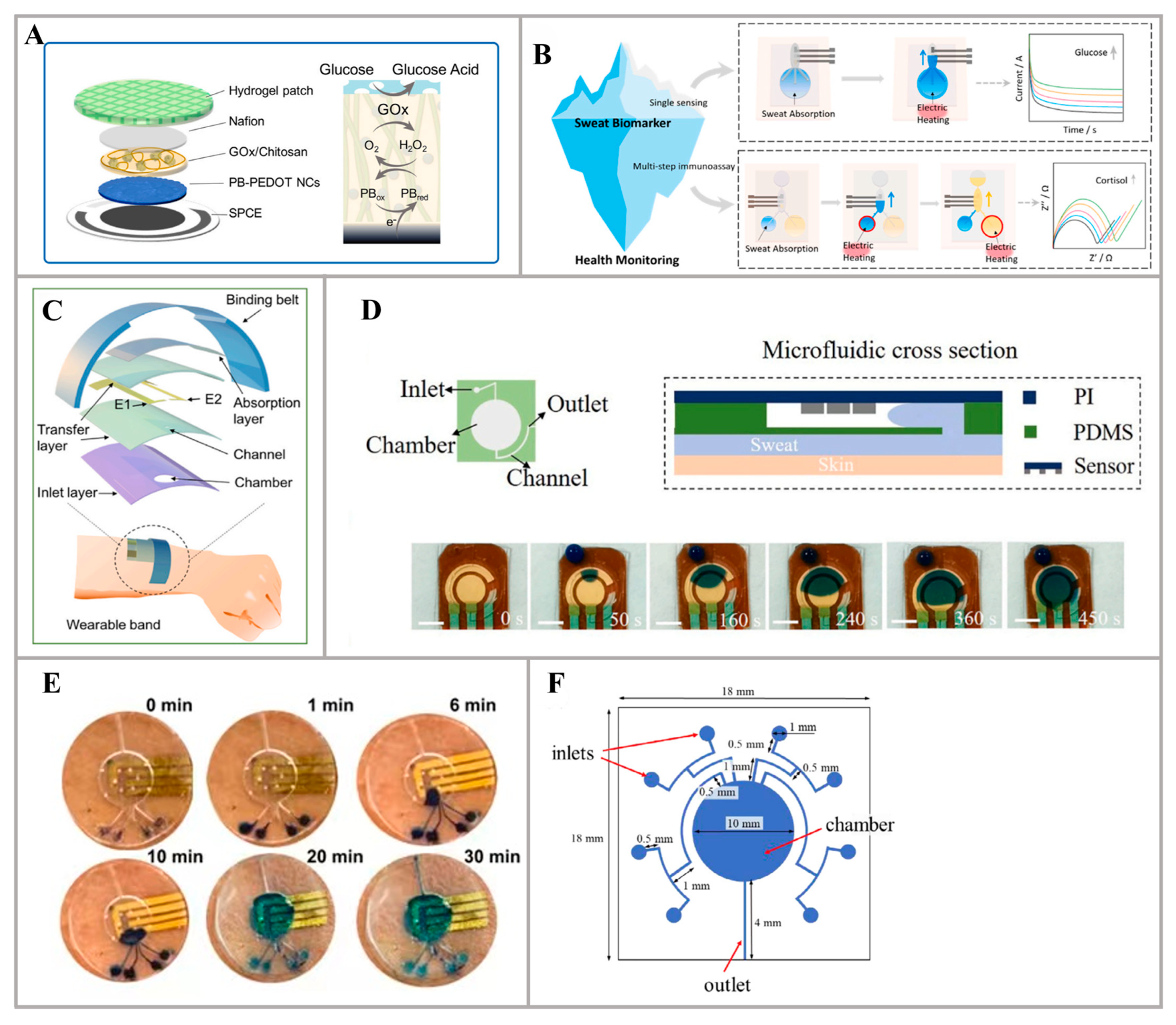

2.2. Innovative Materials for Sweat Detection

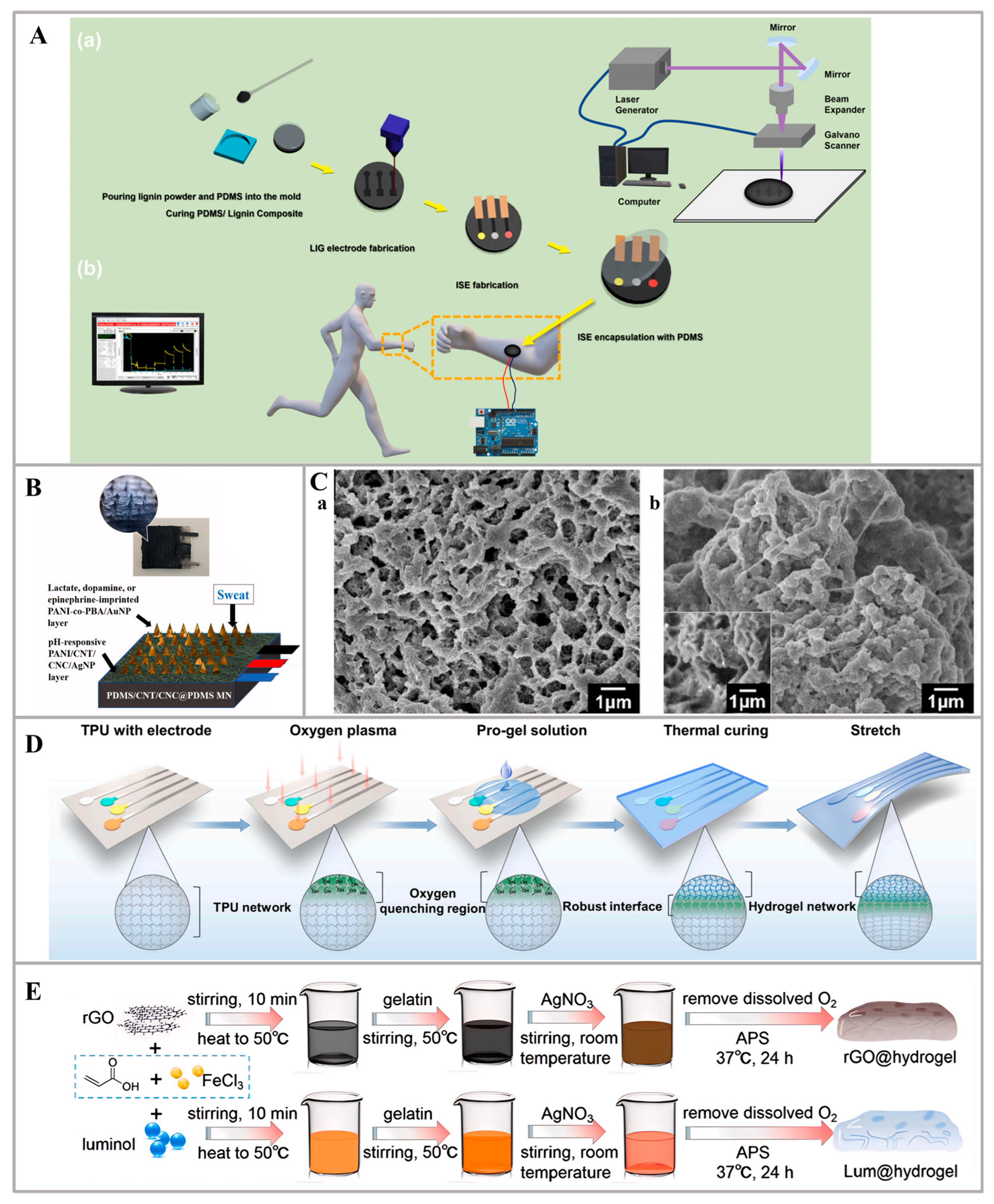
2.3. Innovative Materials for Sweat Sensor Self-Powering

2.4. Others
3. Conclusions and Perspective
- Fabricating materials to ensure comfort, power efficiency, and sensitivity. Comfort and user experience are critical for long-term wearability, necessitating skin-friendly, non-irritating materials that do not disrupt daily activities. Additionally, power efficiency is equally important, with self-powered technologies requiring high efficiency and stable performance to sustain long-lasting operation without frequent charging or battery replacement. Moreover, sweat sensors with high sensitivity and specificity are essential, and capable of detecting a broad range of analytes in sweat. This requires the optimization of materials and detection mechanisms to enable the accurate differentiation and measurement of various biomarkers.
- Sensor performance in stability, durability, miniaturization, and multiplexed sensing systems. Wearable sensors, particularly those utilized in the biomedical field, are subject to stringent demands, primarily regarding their stability and durability. These devices must endure continuous operation without compromising their functionality due to environmental stressors such as varying temperatures, high humidity, and direct sunlight exposure. Furthermore, the challenge of miniaturization without sacrificing functionality is an area of active research. The ability to make sensors smaller while retaining their accuracy and reliability, coupled with their integration into a network of other wearable technology, is key to the future of wearable health monitoring. While miniaturizing, aiming to maximize its functionalities is the objective as well, which can detect different physiological indicators through a single sensor device.
- Personal sensing devices: customization, privacy, and validation. Customization and personalization are paramount due to the unique physiological responses that vary from person to person. Personal sensing devices must therefore be capable of accommodating personalized settings and algorithms to ensure that the information provided to the user is accurate and relevant. Additionally, data privacy and security are of utmost importance considering the sensitive nature of health data collected by these devices. Stringent encryption and robust data protection mechanisms are necessary to prevent unauthorized access and potential misuse of the information. Furthermore, it is essential to establish the accuracy and reliability of personal sensing devices in clinical settings to gain the trust of healthcare providers and enable widespread adoption in medical practice. This validation process involves rigorous testing and verification to ensure that the devices provide trustworthy results.
Author Contributions
Funding
Conflicts of Interest
References
- Schoonen, A.J.M.; Schmidt, F.J.; Hasper, H.; Verbrugge, D.A.; Tiessen, R.G.; Lerk, C.F. Development of a Potentially Wearable Glucose Sensor for Patients with Diabetes Mellitus: Design and In-vitro Evaluation. Biosens. Bioelectron. 1990, 5, 37–46. [Google Scholar] [CrossRef]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef]
- Ates, H.C.; Yetisen, A.K.; Güder, F.; Dincer, C. Wearable devices for the detection of COVID-19. Nat. Electron. 2021, 4, 13–14. [Google Scholar] [CrossRef]
- Bonato, P. Wearable sensors/systems and their impact on biomedical engineering. IEEE Eng. Med. Biol. Mag. 2003, 22, 18–20. [Google Scholar] [CrossRef]
- Zhang, S.; Sunami, Y.; Hashimoto, H. Mini Review: Nanosheet Technology towards Biomedical Application. Nanomaterials 2017, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, C.; Sun, X.; Huang, W. Current development of materials science and engineering towards epidermal sensors. Prog. Mater. Sci. 2022, 128, 100962. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Z.; Zhao, W.; Sun, X.; Xu, L.; Jin, B.; Wang, Q.; Liu, C.; Yang, C. An EMG-based wearable multifunctional Eye-control glass to control home appliances and communicate by voluntary blinks. Biomed. Signal Process. Control 2023, 86, 105175. [Google Scholar] [CrossRef]
- Zhang, S.; Kai, Y.; Sunami, Y. Tactile Sliding Behavior of R2R Mass-Produced PLLA Nanosheet towards Biomedical Device in Skin Applications. Nanomaterials 2018, 8, 210. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, W.; Liu, C.; Zeng, J.; He, Z.; Wang, C.; Yuan, W.; Wang, Q. Flower-like CoO nanowire-decorated Ni foam: A non-invasive electrochemical biosensor for glucose detection in human saliva. Appl. Mater. Today 2024, 36, 102083. [Google Scholar] [CrossRef]
- Guo, S.; Wu, K.; Li, C.; Wang, H.; Sun, Z.; Xi, D.; Zhang, S.; Ding, W.; Zaghloul, M.E.; Wang, C.; et al. Integrated contact lens sensor system based on multifunctional ultrathin MoS2 transistors. Matter 2021, 4, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, D.; Zhang, S.; Dong, Q.; Li, B.; Tran, N.; Li, Z.; Xiong, Y.; Zaghloul, M.E. Development of a Cloud-Based Epidermal MoSe2 Device for Hazardous Gas Sensing. Adv. Funct. Mater. 2019, 29, 1900138. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Luo, S.; Wang, C.; Zhang, S.; Guo, S. Paper-Cut Flexible Multifunctional Electronics Using MoS2 Nanosheet. Nanomaterials 2019, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2019, 92, 378–396. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Heng, W.; Yang, G.; Kim, W.S.; Xu, K. Emerging wearable flexible sensors for sweat analysis. Bio-Des. Manuf. 2021, 5, 64–84. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Ozone, Y.; Morishita, Y.; Matsui, H.; Loew, N.; Motosuke, M.; Mukaimoto, T.; Kobayashi, M.; Mitsuhara, T.; Sugita, Y.; et al. Air-Bubble-Insensitive Microfluidic Lactate Biosensor for Continuous Monitoring of Lactate in Sweat. ACS Sens. 2023, 8, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Qiao, L.; Benzigar, M.R.; Subramony, J.A.; Lovell, N.H.; Liu, G. Advances in Sweat Wearables: Sample Extraction, Real-Time Biosensing, and Flexible Platforms. ACS Appl. Mater. Interfaces 2020, 12, 34337–34361. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Liu, C.; Xu, T.; Wang, D.; Zhang, X. The role of sampling in wearable sweat sensors. Talanta 2020, 212, 120801. [Google Scholar] [CrossRef]
- Lin, P.-H.; Chang, W.-L.; Sheu, S.-C.; Li, B.-R. A Noninvasive Wearable Device for Real-Time Monitoring of Secretion Sweat Pressure by Digital Display. iScience 2020, 23, 101658. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Xia, H.; Tang, H.; Zhou, B.; Li, Y.; Zhang, X.; Shi, Z.; Deng, L.; Song, R.; Li, L.; Zhang, Z.; et al. Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuators B Chem. 2020, 312, 127962. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Liu, J.; Yu, X.; Yang, J.; Li, Y. A simple yet multifunctional sensing platform inspired by healing-assembly hydrogels serving motion and sweat monitoring. Sens. Actuators B Chem. 2023, 378, 133173. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, L.-Y.; Wang, B.-C.; Huang, T.-H.; Shih, M.-J.; Hung, W.-S.; Lai, J.-Y.; Ho, K.-C.; Yeh, M.-H. Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat. Nano Energy 2022, 100, 107464. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, N.; Liu, C.; Ma, B.; Zhang, K.; Li, R.; Wang, Q.; Zhang, S. New Advances in Antenna Design toward Wearable Devices Based on Nanomaterials. Biosensors 2024, 14, 35. [Google Scholar] [CrossRef]
- Lin, K.-C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Luo, D.; Sun, H.; Li, Q.; Niu, X.; He, Y.; Liu, H. Flexible Sweat Sensors: From Films to Textiles. ACS Sens. 2023, 8, 465–481. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef]
- Liu, H.; Gu, Z.; Zhao, Q.; Li, S.; Ding, X.; Xiao, X.; Xiu, G. Printed circuit board integrated wearable ion-selective electrode with potential treatment for highly repeatable sweat monitoring. Sens. Actuators B Chem. 2022, 355, 131102. [Google Scholar] [CrossRef]
- Chang, T.; Li, H.; Zhang, N.; Jiang, X.; Yu, X.; Yang, Q.; Jin, Z.; Meng, H.; Chang, L. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst. Nanoeng. 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Shahub, S.; Upasham, S.; Ganguly, A.; Prasad, S. Machine learning guided electrochemical sensor for passive sweat cortisol detection. Sens. Bio-Sens. Res. 2022, 38, 100527. [Google Scholar] [CrossRef]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A machine learning-based multimodal electrochemical analytical device based on eMoSx-LIG for multiplexed detection of tyrosine and uric acid in sweat and saliva. Anal. Chim. Acta 2022, 1232, 340447. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhong, T.; He, H.; Zhao, T.; Xing, L.; Zhang, Y.; Xue, X. A self-powered wearable sweat-evaporation-biosensing analyzer for building sports big data. Nano Energy 2019, 59, 754–761. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Wang, E.; Liu, M.; Xie, L.; Bai, Y.; Yang, Y.; Xue, J.; Qu, X.; Xi, Y.; Li, L.; et al. A Self-Powered Wearable Sensor for Continuous Wireless Sweat Monitoring. Small Methods 2022, 6, 2200653. [Google Scholar] [CrossRef] [PubMed]
- Veenuttranon, K.; Kaewpradub, K.; Jeerapan, I. Screen-Printable Functional Nanomaterials for Flexible and Wearable Single-Enzyme-Based Energy-Harvesting and Self-Powered Biosensing Devices. Nano-Micro Lett. 2023, 15, 85. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-powered and wearable biosensors for healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Yang, H.; Wang, R.; Wu, W. Roadmap on bio-derived materials for wearable triboelectric devices. Mater. Today Sustain. 2022, 20, 100219. [Google Scholar] [CrossRef]
- Yang, Q.; Rosati, G.; Abarintos, V.; Aroca, M.A.; Osma, J.F.; Merkoçi, A. Wearable and fully printed microfluidic nanosensor for sweat rate, conductivity, and copper detection with healthcare applications. Biosens. Bioelectron. 2022, 202, 114005. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hui, J.; Zhou, L.; Lin, B.; Sun, H.; Bai, Y.; Zhao, J.; Mao, H. A low-cost and simple-fabricated epidermal sweat patch based on “cut-and-paste” manufacture. Sens. Actuators B Chem. 2022, 368, 132184. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, S.; Ning, Q.; Li, T.; Xu, H.; Sun, Q.; Cui, D.; Wang, K. Dual-signal readout paper-based wearable biosensor with a 3D origami structure for multiplexed analyte detection in sweat. Microsyst. Nanoeng. 2023, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, J.; Li, Z.; Hu, M.; Bian, C.; Lin, S. All fabric and flexible wearable sensors for simultaneous sweat metabolite detection and high-efficiency collection. Talanta 2023, 260, 124610. [Google Scholar] [CrossRef]
- Wang, Z.; Shin, J.; Park, J.H.; Lee, H.; Kim, D.H.; Liu, H. Engineering Materials for Electrochemical Sweat Sensing. Adv. Funct. Mater. 2020, 31, 2008130. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Shi, Y.; Yang, X.; Li, L.; Lu, Q.; Zheng, H.; Feng, S.; Bai, Y.; Zhang, T. Vertically aligned conductive metal-organic framework nanowires array composite fiber as efficient solid-contact for wearable potentiometric sweat sensing. Sens. Actuators B Chem. 2022, 369, 132290. [Google Scholar] [CrossRef]
- Criscuolo, F.; Ny Hanitra, I.; Aiassa, S.; Taurino, I.; Oliva, N.; Carrara, S.; De Micheli, G. Wearable multifunctional sweat-sensing system for efficient healthcare monitoring. Sens. Actuators B Chem. 2021, 328, 129017. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B Chem. 2021, 343, 130131. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, R.; Xu, Z.; Chi, H.; Wang, Z.; Zhao, Y. Directional sweat transport window based on hydrophobic/hydrophilic Janus fabric enables continuous transfer and monitoring of sweat. Appl. Mater. Today 2022, 29, 101623. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, B.; Zuo, Y.; Chen, G.; Hao, Q.; Zhao, C.; Liu, H. Versatile sweat bioanalysis on demand with hydrogel-programmed wearables. Biosens. Bioelectron. 2023, 235, 115412. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT:PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Yang, X.; Lu, Q.; Xiong, Z.; Li, L.; Zheng, H.; Feng, S.; Zhang, T. An unconventional vertical fluidic-controlled wearable platform for synchronously detecting sweat rate and electrolyte concentration. Biosens. Bioelectron. 2022, 210, 114351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Luo, Y.; Su, L.; Lu, J.; Han, W.; Xu, T.; Zhang, X. Hydrophilic metal-organic frameworks integrated uricase for wearable detection of sweat uric acid. Anal. Chim. Acta 2022, 1208, 339843. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2T MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous sweat lactate monitoring system with integrated screen-printed MgO-templated carbon-lactate oxidase biosensor and microfluidic sweat collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Yu, X.; Peng, Z.; Zhang, G.; Li, Y. Wearable molecularly imprinted electrochemical sensor with integrated nanofiber-based microfluidic chip for in situ monitoring of cortisol in sweat. Sens. Actuators B Chem. 2023, 381, 133451. [Google Scholar] [CrossRef]
- Wei, L.; Lv, Z.; He, Y.; Cheng, L.; Qiu, Y.; Huang, X.; Ding, C.; Wu, H.; Liu, A. In-situ admittance sensing of sweat rate and chloride level in sweat using wearable skin-interfaced microfluidic patch. Sens. Actuators B Chem. 2023, 379, 133213. [Google Scholar] [CrossRef]
- Mirzajani, H.; Abbasiasl, T.; Mirlou, F.; Istif, E.; Bathaei, M.J.; Dağ, Ç.; Deyneli, O.; Yazıcı, D.; Beker, L. An ultra-compact and wireless tag for battery-free sweat glucose monitoring. Biosens. Bioelectron. 2022, 213, 114450. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Haque, M.N.; Kabiraz, D.C.; Yeasin, A.; Rashid, H.A.; Sarker, A.C.; Hossain, G. A review on advanced nanocomposites materials based smart textile biosensor for healthcare monitoring from human sweat. Sens. Actuators A Phys. 2023, 350, 114093. [Google Scholar] [CrossRef]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Chu, H.; Hu, X.; Lee, C.-Y.; Zhang, A.; Ye, Y.; Wang, Y.; Chen, Y.; Yan, X.; Wang, X.; Wei, J.; et al. A wearable electrochemical fabric for cytokine monitoring. Biosens. Bioelectron. 2023, 232, 115301. [Google Scholar] [CrossRef] [PubMed]
- Piper, A.; Öberg Månsson, I.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A disposable, wearable, flexible, stitched textile electrochemical biosensing platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef] [PubMed]
- Teekayupak, K.; Lomae, A.; Agir, I.; Chuaypen, N.; Dissayabutra, T.; Henry, C.S.; Chailapakul, O.; Ozer, T.; Ruecha, N. Large-scale fabrication of ion-selective electrodes for simultaneous detection of Na+, K+, and Ca2+ in biofluids using a smartphone-based potentiometric sensing platform. Microchim. Acta 2023, 190, 237. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Zhou, K.; Li, S.; Li, H.; Xu, K.; Tang, H.; Deng, H.; Zhou, Q.; Pan, J.; et al. Screen printing and laser-induced flexible sensors for the simultaneous sensitive detection of uric acid, tyrosine, and ascorbic acid in sweat. Analyst 2023, 148, 2965–2974. [Google Scholar] [CrossRef]
- Lee, C.-W.; Jeong, S.-Y.; Kwon, Y.-W.; Lee, J.-U.; Cho, S.-C.; Shin, B.-S. Fabrication of laser-induced graphene-based multifunctional sensing platform for sweat ion and human motion monitoring. Sens. Actuators A Phys. 2022, 334, 113320. [Google Scholar] [CrossRef]
- Mugo, S.M.; Robertson, S.V.; Lu, W. A molecularly imprinted electrochemical microneedle sensor for multiplexed metabolites detection in human sweat. Talanta 2023, 259, 124531. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Fan, C.; Xu, T.; Zhang, X. Skin-like hydrogel-elastomer based electrochemical device for comfortable wearable biofluid monitoring. Chem. Eng. J. 2023, 455, 140609. [Google Scholar] [CrossRef]
- Ryu, J.; Landers, M.; Choi, S. A sweat-activated, wearable microbial fuel cell for long-term, on-demand power generation. Biosens. Bioelectron. 2022, 205, 114128. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, S. Bioelectricity production from sweat-activated germination of bacterial endospores. Biosens. Bioelectron. 2021, 186, 113293. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Sun, C.; Zhao, X.; Jiao, C.; Du, A.; Wang, Q.; Mao, Y.; Liu, B. Wearable biosensors for real-time sweat analysis and body motion capture based on stretchable fiber-based triboelectric nanogenerators. Biosens. Bioelectron. 2022, 205, 114115. [Google Scholar] [CrossRef] [PubMed]
- Baro, B.; Khimhun, S.; Das, U.; Bayan, S. ZnO based triboelectric nanogenerator on textile platform for wearable sweat sensing application. Nano Energy 2023, 108, 108212. [Google Scholar] [CrossRef]
- Li, H.; Chang, T.; Gai, Y.; Liang, K.; Jiao, Y.; Li, D.; Jiang, X.; Wang, Y.; Huang, X.; Wu, H.; et al. Human joint enabled flexible self-sustainable sweat sensors. Nano Energy 2022, 92, 106786. [Google Scholar] [CrossRef]
- Sun, M.; Gu, Y.; Pei, X.; Wang, J.; Liu, J.; Ma, C.; Bai, J.; Zhou, M. A flexible and wearable epidermal ethanol biofuel cell for on-body and real-time bioenergy harvesting from human sweat. Nano Energy 2021, 86, 106061. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y.; Liu, W.; Li, S.; Wang, X.; Zhou, W.; Zhang, G.; Liu, K.; Zhang, H.; Zhao, Y.; et al. Untethered artificial muscles powered by wearable sweat-based energy generator. Nano Today 2023, 49, 101765. [Google Scholar] [CrossRef]
- Ju, J.; Xiao, G.; Jian, Y.; Wu, L.; Sun, W.; Wang, W.; Li, C.M.; Qiao, Y.; Lu, Z. Scalable, high-performance, yarn-shaped batteries activated by an ultralow volume of sweat for self-powered sensing textiles. Nano Energy 2023, 109, 108304. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Zhou, J.; Li, J.; Nejad, S.K.; Yiu, C.K.; Li, H.; Wong, T.H.; Park, W.; Yao, K.; et al. Bandage based energy generators activated by sweat in wireless skin electronics for continuous physiological monitoring. Nano Energy 2022, 92, 106755. [Google Scholar] [CrossRef]
- Wang, S.; Bai, Y.; Yang, X.; Liu, L.; Li, L.; Lu, Q.; Li, T.; Zhang, T. Highly stretchable potentiometric ion sensor based on surface strain redistributed fiber for sweat monitoring. Talanta 2020, 214, 120869. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Wu, J.; Shu, Q.; Wang, D.; Li, M.; Liu, D.; Wang, X.; Lei, W. High gain fiber-shaped transistor based on rGO-mediated hierarchical polypyrrole for ultrasensitive sweat sensor. Sens. Actuators A Phys. 2023, 354, 114297. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Gyu Son, S.; Jun Park, H.; Kim, S.-M.; Jin Kim, S.; Sik Kil, M.; Jeong, J.-M.; Lee, Y.; Eom, Y.; Yeon Hwang, S.; Park, J.; et al. Ultra-fast self-healable stretchable bio-based elastomer/graphene ink using fluid dynamics process for printed wearable sweat-monitoring sensor. Chem. Eng. J. 2023, 454, 140443. [Google Scholar] [CrossRef]
| Material Type | Method | Analyte | Detection Range | Sensitivity | Ref. | |
|---|---|---|---|---|---|---|
| Innovative materials for sweat collection | PDMS and nanofiber films | MIP | cortisol | 1 nM–1 μM | [70] | |
| PDMS | CA | uric acid | 2–250 µM, LOD: 1.2 μM | 0.875 µA/µM/cm2 | [64] | |
| PDMS | EIS | sweat rate and total electrolyte concentration | Sweat rate: 0.5–20 μL/min/cm2, Total electrolyte concentration: 1–200 mM | [65] | ||
| PDMS | CA | uric acid | 2–70 μM, LOD: 0.34 μM | [66] | ||
| PDMS | EIS | cortisol | 0.01–100 nM, LOD:1 pg/mL | [67] | ||
| PDMS | DPV | cortisol | 1 pM to 1 μM, LOD 0.2 pM | [68] | ||
| PDMS | CA | lactate | 0–10 mM | 36.2 μA/mM/cm2 | [69] | |
| PDMS | CA | glucose and lactate | [53] | |||
| hydrogel | CA | glucose | 6.25 μM to 0.8 mM | [62] | ||
| hydrogel | CA, EIS | glucose and cortisol | Glucose: 5.0–50 μM, Cortisol: 1.0–15 ng/mL | [63] | ||
| paper | MIP | cortisol | 1 nM–1 μM | [55] | ||
| paper | CA | glucose and lactate | Glucose: 0.08–1.25 mM, Lactate: 0.3–20.3 mM | Glucose: 2.4 nA/μM, Lactate: 0.49 μA/mM | [52] | |
| cloth | CA | glucose | 0.05–1 mM | 105.93 μA/mM/cm2 | [60] | |
| Silicone and PET | EIS | sweat rate and chloride | [71] | |||
| PET | EIS | sweat rate, conductivity, and copper levels | 0–2500 ng/mL, LOD: 396 ng/mL | 2.3 nA/ng/mL | [51] | |
| PET | CA | glucose | 0.1–1 mM, LOD: 24 μM | 1.27 μA/mM/cm2 | [72] | |
| fabric with Janus structure | CA, CP | glucose, Na+, K+, NH4+, and pH levels | Glucose: 0–500 µM, NaCl: 10–160 mM, KCl: 2–32 mM, NH4Cl: 10–160 mM, pH: 4–7 | Glucose: 3.53 nA/µM, Na+: 48.4 mV/decade, K+: 51.2 mV/decade, NH4+: 53.9 mV/decade, pH: 47.8 mV/decade | [61] | |
| fabric with Janus structure | OCP, CA | Na+, pH, and glucose | Na+: 5–160 mM, pH: 4 to 8 | Na+: 53.83 mV/decade, pH: 98.68 mV/decade, glucose: 0.04 μA/μM | [56] | |
| cotton | CP | Li+, Pb2+, K+ and Na+ | LOD of Li+: 1.4 ± 0.2 mM, LOD of Pb2+: 4.13 ± 0.23 mM, LOD of K+: 3.10 ± 0.10 mM, LOD of Na+: 14.30 ± 5.13 µM | Li+: 57.6 ± 2.1 mV/decade, Pb2+: 58.8 ± 1.4 mV/decade, K+: 55.1 ± 0.9 mV/decade, Na+: 28.9 ± 1.64 mV/decade | [59] | |
| Innovative materials for sweat detection | hydrophobic paper | EIS, CP, OCP | K+, Na+, Cl−, and pH | [75] | ||
| fiber/fabric | SWV | IL-6 | 1 pg/mL to 100 ng/mL, LOD: 280 fg/mL | [76] | ||
| fiber/fabric | CV | glucose | 0.1–0.6 mM, LOD: 301 ± 2 nM | 126 ± 14 nA/mM | [77] | |
| PVC, PI, and PET | CP | K+, Na+, and Ca2+ | LOD of K+: 10 mM, LOD of Na+: 10 mM, LOD of Ca2+: 100 mM | [78] | ||
| PI | CV, DPV | uric acid (UA), tyrosine (Tyr), and ascorbic acid (AA) | UA, AA and Tyr: 10–160 μM. LODs of UA: 0.47 μM, LOD of AA: 1.25 μM, LOD of Tyr: 14.38 μM | [79] | ||
| CNT-EVA film | CV, CA | glucose | LOD: 3 μM | 270 ± 10 μA/mM/cm2 | [34] | |
| PDMS and lignin-LIG | CP | Na+ and K+ | Na+: 0.1 μM to 0.1 M, K+: 0.01 μM to 0.1 M | Na+: 63.6 mV/decade, K+: 59.2 mV/decade | [80] | |
| multilayer PDMS | MIPs | pH, epinephrine, dopamine, and lactate | pH: 4.25–10, LOD of epinephrine: 0.7 ± 0.2 nM, LOD of dopamine: 2.11 ± 0.05 nM, LOD of lactate: 0.07 ± 0.07 mM | [81] | ||
| conductive hydrogel | OCP, DPV, CV | pH and tyrosine (Tyr) | Tyr: 10–200 μM (R2 = 0.9985), LOD: 3.3 μM | pH: −71.86 mV/pH | [82] | |
| hydrogel and TPU | OCP | pH, Na+, K+ | pH: 4–9, Na+: 5–160 mM, K+: 1–32 mM | pH: 58.14 mV/pH, Na+: 58.89 mV/decade, K+: 59.11 mV/decade | [83] | |
| rGO@ hydrogel and Lum@ hydrogel | ECL | urea, lactic acid, and chlorion | [35] |
| Material Type | Method | Generator Size | Voltage (V) | Analyte | Power Density | Ref. |
|---|---|---|---|---|---|---|
| PVDF/graphene and poly (3-aminophenyl boronic acid) (3-APBA) | triboelectric nanogenerator (TENG) | Output voltage of 30 V | Lactate | [36] | ||
| MWCNTs/polyaniline (PANI)/Ecoflex fiber, and twining varnished wires | fiber-based triboelectric nanogenerators (F-TENGs) | 2 mm (diameter) | Open-circuit voltage of 3 V | Glucose, creatinine and lactate acid | [86] | |
| Teflon (PTFE)–ZnO | Single-electrode triboelectric nanogenerator (STENG) | 10 mm (diameter) | Open-circuit voltage of 5 V | Cl ions | 2.5 µW/cm2 | [87] |
| PET, Ag, and PVDF | piezoelectric nanogenerators (PENGs) | 40 mm × 15 mm | Output voltage of 97 V | Na+, K+, and pH | 140 mW/m2 | [88] |
| Carbon film@LOx | water-evaporation nanogenerator | 25 mm × 25 mm | Output voltage of 0.2 V | Lactate | [45] | |
| NQ/MWCNT; PB/MWCNT | enzymatic biofuel cell (BFC) | 35 mm × 15 mm | Open-circuit voltage of 0.45 V | Glucose | 266 μW/cm2 | [48] |
| 3D-NHCAs, alcohol oxidase (AOx), bilirubin oxidase (BOx), and TPA | ethanol biofuel cell | 40 mm × 45 mm | Ethanol | 1.9 μW/cm2 | [89] | |
| SWCNTs@Cu; zinc foil | redox reactions | 50 mm (diameter) | Output voltage of 0.8 V | EMG | 18.3 μw/cm2 | [90] |
| Zn-wire core, cotton yarn, and carbon yarn | redox reactions | 1.6 mm (diameter) | Output voltage of 0.73 V | The arm swing frequency and breathing rate | 1.72 mW/cm2 | [91] |
| Cotton, Mg, cotton/KCl, Ni/G Foam | redox reactions | 40 mm × 20 mm | Open-circuit voltage of 1.4 V | temperature, pulse rate (PR), and oxygen saturation in blood (SpO2) | 16.3 mW/cm2 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; He, Z.; Zhao, W.; Liu, C.; Zhou, S.; Ibrahim, O.O.; Wang, C.; Wang, Q. Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications. Nanomaterials 2024, 14, 857. https://doi.org/10.3390/nano14100857
Zhang S, He Z, Zhao W, Liu C, Zhou S, Ibrahim OO, Wang C, Wang Q. Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications. Nanomaterials. 2024; 14(10):857. https://doi.org/10.3390/nano14100857
Chicago/Turabian StyleZhang, Sheng, Zhaotao He, Wenjie Zhao, Chen Liu, Shulan Zhou, Oresegun Olakunle Ibrahim, Chunge Wang, and Qianqian Wang. 2024. "Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications" Nanomaterials 14, no. 10: 857. https://doi.org/10.3390/nano14100857





