Effect of Scanning Electron Beam Pretreatment on Gas Carburization of 22CrMoH Gear Steel
Abstract
:1. Introduction
2. Implementation of the Research
2.1. Experimental Materials and Methods
2.2. Numerical Simulation Model of the Scanning Electron Beam
3. Analysis of Experimental Results
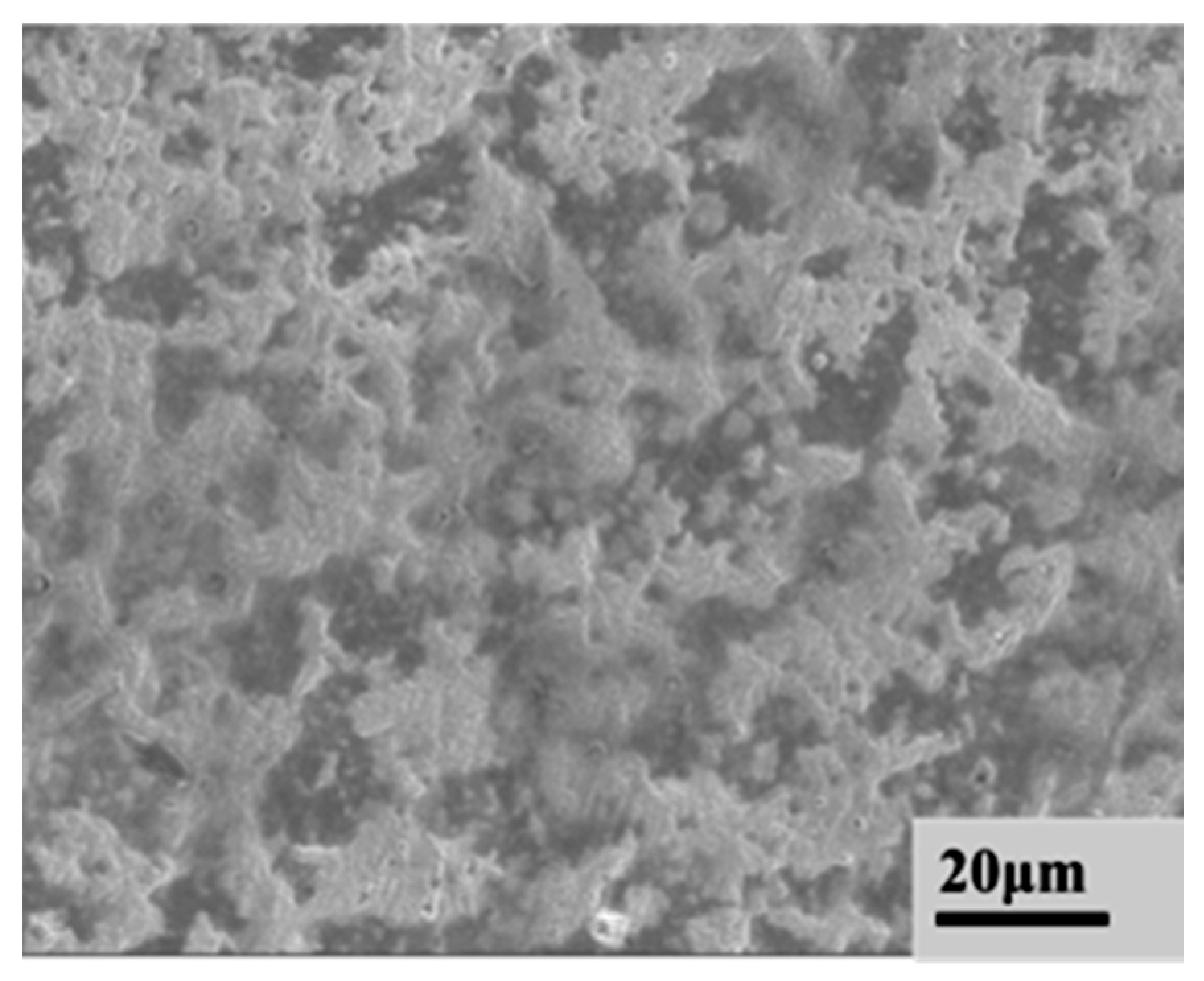
3.1. Surface Morphology and Surface Roughness of Scanned Electron Beam Layers
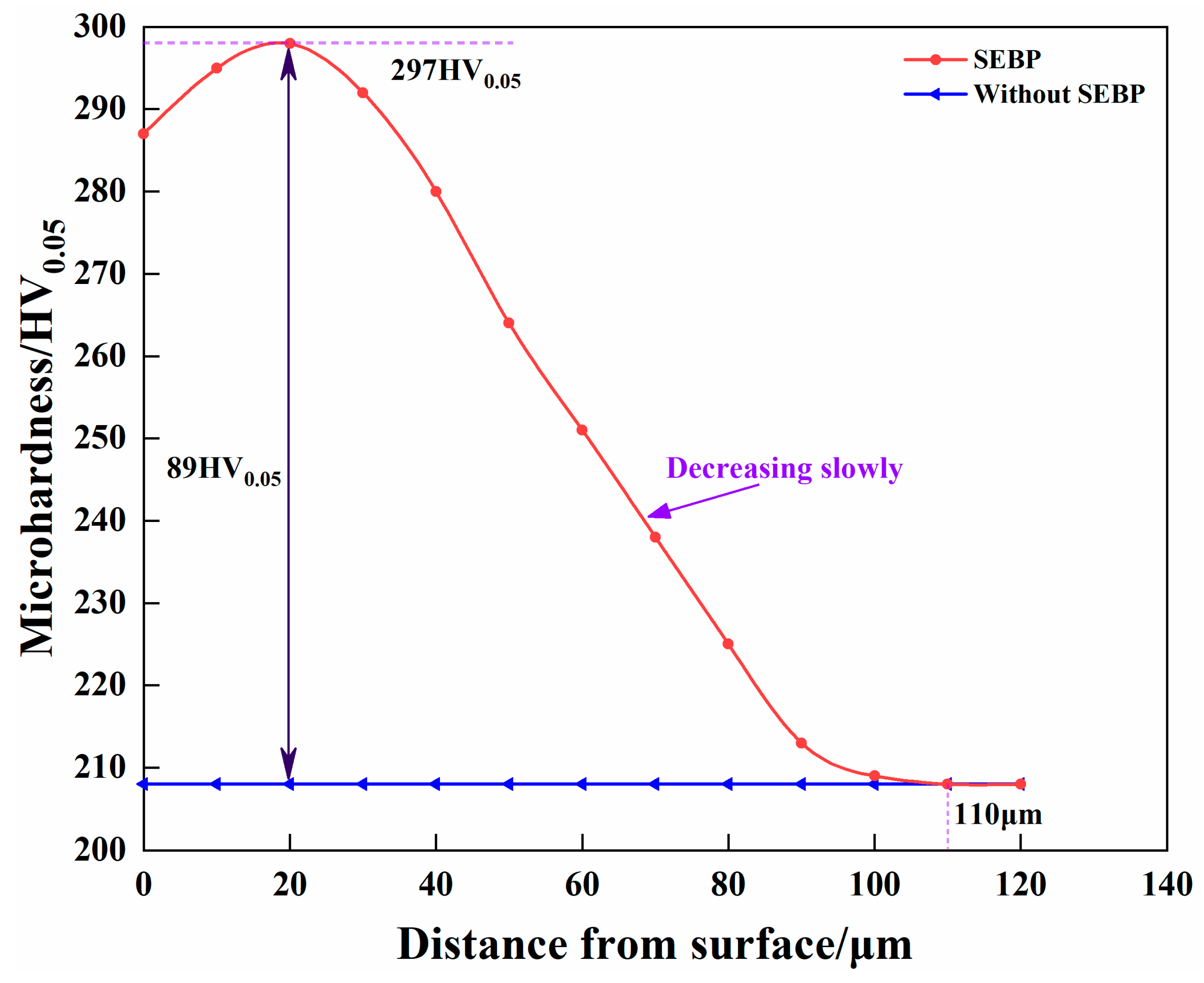
3.2. Cross-Sectional Hardness after SEBP
3.3. Microstructure Analysis
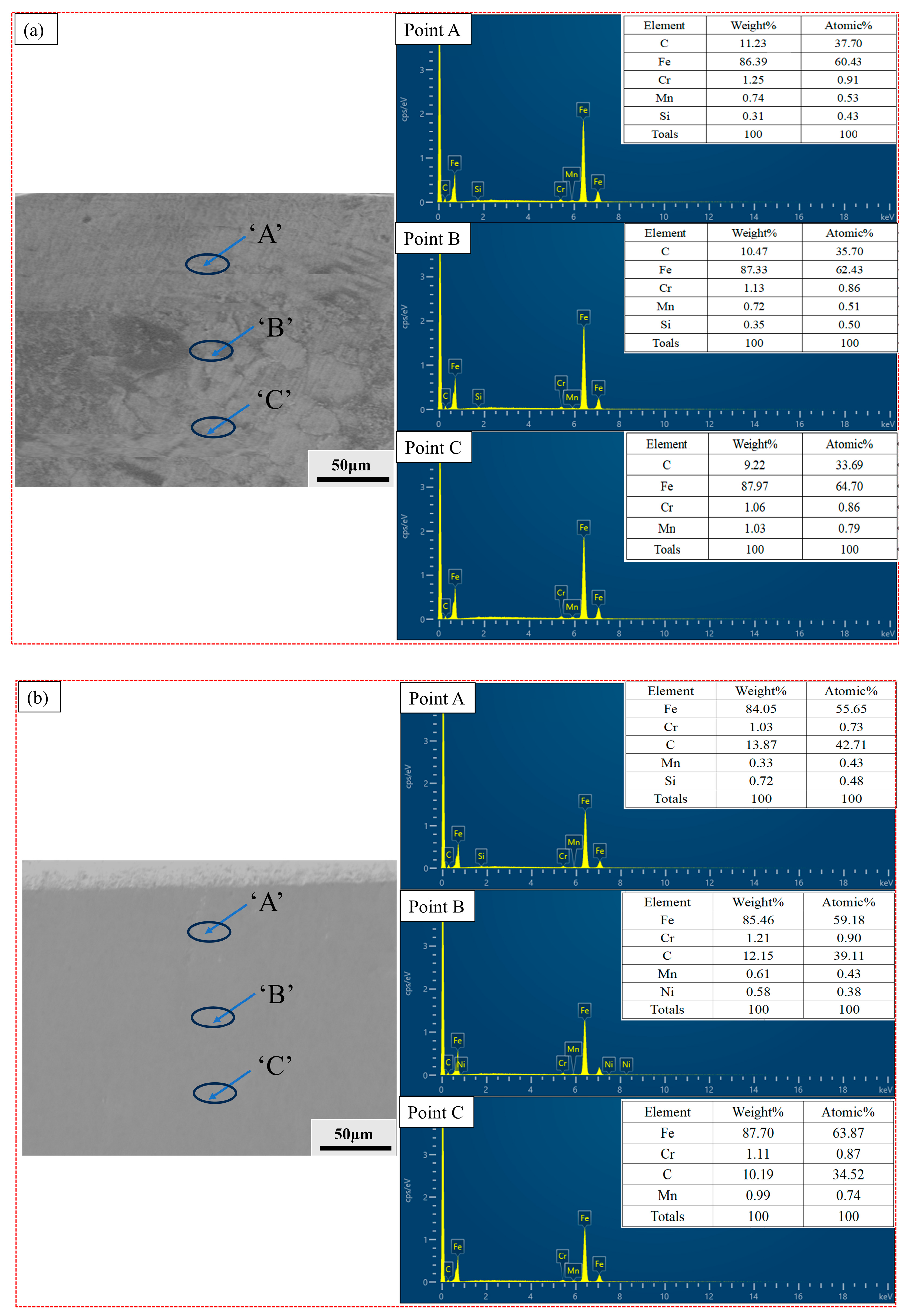
3.4. XRD and Energy-Dispersive Spectroscopy (EDS) Analysis
3.5. Effect of Microhardness and Wear Resistance
3.6. Enhanced Carburization and Strengthening Mechanism Analysis
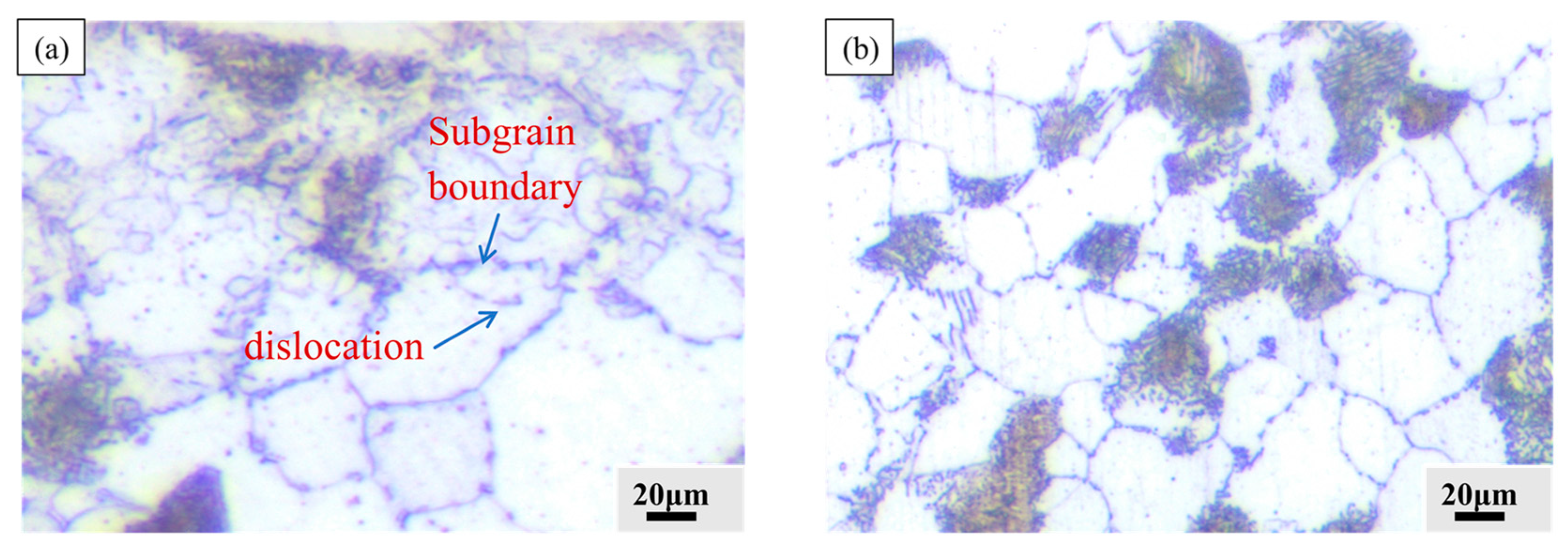
- The SEBP formed a 110 μm thick plastic deformation layer on the surface of the specimen. As shown in Figure 15a, numerous crystal defects, especially dislocations and subcrystalline boundaries, are found in the deformation layer. The rate of diffusion of active carbon atoms along the crystal defects was substantially higher than that in the body. Therefore, the crystal defects formed by the plastic deformation layer of the surface are conducive to the rapid diffusion of active carbon atoms adsorbed on the surface into the substrate [39,40]. In the process of gas carburization, the formation of the carburized layer mainly depended on the surface adsorption and inward diffusion of activated carbon atoms, so that the surface layer had high carburization and carbon concentration.
4. Conclusions
- (1)
- The SEBP increased the surface roughness of the specimen from 0.182 to 4.676 μm, which was conducive to the adsorption of active carbon atoms on the surface during gas carburization. In addition, a deformation layer with a thickness of approximately 110 μm was formed on the specimen after SEBP, and the microhardness of the cross-sectional surface was improved, which aided the inward diffusion of carbon atoms during carburization.
- (2)
- The scanning electron beam improved the gas-carburizing efficiency, and under the same conditions, the grain refinement effect was more evident, the properties of the tissue were improved, the thickness of the carburized layer increased from 0.78 to 1.09 mm, and the carburizing efficiency improved by 39%.
- (3)
- The Fe-C phase diffraction peaks of the carburized sample with SEBP were enhanced. The EDS elemental analysis showed that the tissue morphology changed significantly during the SEBP, and carbon aggregation appeared in the scanned layer.
- (4)
- The GC specimen with the SEBP had a higher cross-sectional hardness gradient. Its friction coefficient was reduced from approximately 0.8 μ to almost 0.45 μ, and the wear amount of the specimens with SEBP was 47.7% of that of the matrix specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, M.A.; Asgar, H.; Abdullah, A.; RAhmad, R.; Inam, A.; Ghauri, F.A.; Ghauri, F.A. Carburising of Low-Carbon Steel Using Carbon Black Nanoparticles. Arab. J. Sci. Eng. 2016, 41, 4661–4667. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Kumar, S.; Sarangi, S. TVMS calculation and dynamic analysis of carburized spur gear pair. Mech. Syst. Signal Process. 2022, 166, 108436. [Google Scholar] [CrossRef]
- Asahi, N.; Takeuchi, H.; Funaki, Y.; Pin, K.K.; Urao, R. Effect of gas flow on ion carburizing of steel. J. Phase Equilibria Diffus. 2009, 30, 235–241. [Google Scholar]
- Wolowiec-Korecka, E.; Korecki, M.; Sut, M.; Brewka, A.; Kula, P. Calculation of the Mixture Flow in a Low-Pressure Carburizing Process. Metals 2019, 9, 439. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, K.H. Effects of Carburizing Process on Sliding wear Behavior of Carburized SCM420H Steel. Tribol. Lubr. 2020, 36, 18–26. [Google Scholar]
- Tang, L.; Mao, C.J.; Jia, W.J.; Wei, K.X. The effect of novel composite pretreatment on performances of plasma nitrided layer. J. Mater. Res. Technol. 2020, 9, 9531–9536. [Google Scholar] [CrossRef]
- Yu, T.B.; Li, C.; Zhang, D.X.; Wang, W.S.; Zhang, Y.F.; Butle, D.L.; Wang, J.; Yui, A.; Zhou, L. Study on the Simulation of Grinding Burn. Adv. Abras. Technol. XV 2012, 565, 58–63. [Google Scholar] [CrossRef]
- Miao, B.; Chai, Y.T.; Wei, K.X.; Hu, J. A novel duplex plasma treatment combining plasma nitrocarburizing and plasma nitriding. Vacuum 2016, 13, 54–57. [Google Scholar] [CrossRef]
- Tang, L.; Jia, W.J.; Hu, J. An enhanced rapid plasma nitriding by laser shock peening. Mater. Lett. 2018, 231, 91–93. [Google Scholar] [CrossRef]
- Xu, J.L.; Yang, W.L.; Niu, L.; Dan, X.M.; Kang, C.H.; Ma, B.X. The Research Progress of Nanoparticles Surface Modification by Using Mechanochemical Method. In Micro-Nano Technology XVII-XVIII, Proceedings of the 17th–18th Annual Conference and 6th–7th International Conference of the Chinese Society of Micro-Nano Technology; World Scientific: Singapore, 2018; pp. 250–255. [Google Scholar]
- Khun, N.W.; Trung, P.Q.; Butler, D.L. Mechanical and Tribological Properties of Shot-Peened SAE 1070 Steel. Tribol. Trans. 2016, 59, 932–943. [Google Scholar] [CrossRef]
- Yu, J.; Wang, R.; Wei, D.Q.; Meng, C.G. Effect of different scanning modes on the surface properties of continuous electron beam treated 40CrMn steel. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 467, 102–107. [Google Scholar] [CrossRef]
- Valkov, S.; Ormanova, M.; Petrov, P. Electron-Beam Surface Treatment of Metals and Alloys. Tech. Trends Met. 2020, 10, 1219. [Google Scholar]
- Matsumoto, T.; Okumura, Y.; Ichimura, M.; Nakamura, H.; Honda, K.; Shibahara, M.; Hirano, T.; Yamada, H.; Tanaka, R.; Sakai, I.; et al. Surface Modification and Adhesion Mechanism of Isotactic Polypropylene with Low-Energy Electron-Beam Treatments. Langmuir 2020, 36, 10846–10852. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Kim, J.; Park, S.S.; Park, H.W.; Ki, H. Surface modification of the patterned Al6061/SUS304 metal plates using the large electron beam. Appl. Surf. Sci. 2012, 261, 458–463. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.S.; Kang, E.G.; Park, H.W. Surface modification of the metal plates using continuous electron beam process (CEBP). Appl. Surf. Sci. 2014, 311, 201–207. [Google Scholar] [CrossRef]
- Zhao, G.H.; Zhang, Y.; Zhao, H.; Li, J.; Li, H.Y.; Ma, L.F. Study on the process optimization and wear resistance of electron beam cladding AlCoCrFeNi coating on 253MA austenitic stainless steel surface. Mater. Charact. 2023, 205, 113341. [Google Scholar] [CrossRef]
- Valkov, S.; Parshorov, S.; Andreeva, A.; Rabadzhiyska, S.; Nikolova, M.; Bezdushnyi, R.; Petrov, P. Influence of beam power on the surface architecture and corrosion behavior of electron-beam treated Co-Cr-Mo alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 494–495, 46–52. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, S.; He, G.N.; Jiang, B.; Zhou, L.Y.; Chen, Y.; Liu, Y.Z. Failure analysis of the carburized 20MnCr5 gear in fatigue working condition. Int. J. Fatigue 2022, 161, 10938. [Google Scholar] [CrossRef]
- Ye, X.M.; Wu, J.Q.; Zhu, Y.L.; Hu, J. On the role of chemical banding and austenite grain size in microstructure evolutions and phase transformation kinetics of gear steels. Vacuum 2014, 110, 74–744. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Zuo, X.; Yu, W.; Qiu, L.; Luo, Z.; Sun, J. On the role of chemical banding and austenite grain size in microstructure evolutions and phase transformation kinetics of gear steels. Constr. Build. Mater. 2022, 356, 129305. [Google Scholar] [CrossRef]
- Abdi, R.; Tunç, C. Pseudo symmetry curvature conditions on submanifolds of conformal Kenmotsu manifolds. Nonlinear Stud. 2022, 29, 457–476. [Google Scholar]
- Pavelic, V. Experiment and computed temperature histories in gas tungsten-arc welding of thin plates. Wdlding Jourmal Res. Suppl. 1968, 48, 206–305. [Google Scholar]
- Song, L.; Gu, X.M.; Sun, F.; Hu, J. Reduced internal oxidation by a rapid carburizing technology enhanced by pre-oxidation for 18CrNiMo7-6 gear steel. Vacuum 2019, 160, 210–212. [Google Scholar] [CrossRef]
- Chen, X.D.; Feng, S.; Wang, L.W.; Tang, R.; Zhang, F.; Ming, S.L.; Cai, Z.B. Effect of QPQ on the fretting wear behavior of TP316H steel at varying temperatures in liquid sodium. J. Nucl. Mater. 2022, 562, 153583. [Google Scholar] [CrossRef]
- Wang, S.K.; Cai, W.; Li, J.C.; Wei, W.; Hu, J. A novel rapid DC plasma nitriding at low gas pressure for 304 austenitic stainless steel. Mater. Lett. 2013, 105, 47–49. [Google Scholar] [CrossRef]
- Peng, T.T.; Dai, M.Y.; Cai, W.; Wei, W.; Wei, K.X.; Hu, J. The enhancement effect of salt bath preoxidation on salt bath nitriding for AISI 1045 steel. Appl. Surf. Sci. 2019, 484, 610–615. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.Q.; Miao, B.; Zhao, X.B.; Mao, C.J.; Wei, W.; Hu, J. Enhancement of wear resistance by sand blasting-assisted rapid plasma nitriding for 304 austenitic stainless steel. Surf. Eng. 2019, 36, 524–530. [Google Scholar] [CrossRef]
- Batalha, G.F.; Silva, L.C.; Coelho, R.S. Mechanical properties characterization of Ti-6Al-4 V grade 5 (recycled) additively manufactured by selective electron beam melting (EB-PBF). Eng. Fail. Anal. 2024, 157, 107892. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wei, J.L.; Liu, T.; Liu, S.; Fang, D.L.; Li, Z.Q. Influence of carbon layer hardness characteristics on RCF performance of carburized bearing steel and optimization of heat treatment process. Eng. Fail. Anal. 2024, 160, 108164. [Google Scholar] [CrossRef]
- Xiao, N.; Hui, W.J.; Zhang, Y.J.; Zhao, X.L.; Chen, Y.; Dong, H. High cycle fatigue behavior of a low carbon alloy steel: The influence of vacuum carburizing treatment. Eng. Fail. Anal. 2020, 109, 104215. [Google Scholar] [CrossRef]
- Gupta, A.; Bennett, C.J.; Sun, W. The role of defects and characterisation of tensile behaviour of EBM Additive manufactured Ti-6Al-4V: An experimental study at elevated temperature, Engineering. Fail. Anal. 2021, 120, 105115. [Google Scholar] [CrossRef]
- Dwivedi, P.K.; Sivateja, C.; Rai, A.K.; Ganesh, P.; Basu, A.; Dutta, K. A comparative assessment of the effects of laser shock peening and ultrasonic shot peening on surface integrity and ratcheting fatigue performance of HSLA steel. Int. J. Fatigue 2023, 176, 107902. [Google Scholar] [CrossRef]
- Jeong, D.H.; Erb, U.; Aust, K.T.; Palumbo, G. The relationship between hardness and abrasive wear resistance of electrodeposited nanocrystalline Ni–P coatings. Scr. Mater. 2003, 48, 1067–1072. [Google Scholar] [CrossRef]
- Li, J.; Men, S.W.; Zhang, Z.; Yang, Y.; Sun, Y.; Ding, J.C.; Wang, Q.M. Structural, mechanical, and tribological properties of GLC film on a nitrided layer prepared in a glow-discharge plasma nitriding system. Vacuum 2021, 193, 110543. [Google Scholar] [CrossRef]
- Hirata, T.; Yamaguchi, T.; Yokoyama, Y.; Hoshino, H. Surface modification by high-speed laser gas carburization in low-alloy steel. Mater. Lett. 2020, 280, 128586. [Google Scholar] [CrossRef]
- Wang, J.T.; Qu, S.G.; Shao, H.T.; Hu, X.F.; Guo, B.; Li, X.Q. Ultra-high fatigue propertyand fracture mechanism of modified 20CrMoH steel by gas carburizing technology combined with shot peening treatment. Int. J. Fatigue 2022, 165, 107221. [Google Scholar] [CrossRef]
- Konno, T.; Kato, T.; Yanagida, T.; Inaba, H.; Hirata, Y.; Akasaka, H.; Ohtake, N. Characterization of carburized layer on low-alloy steel fabricated by hydrogen-free carburizing process using carbon ions. Diam. Relat. Mater. 2023, 135, 109816. [Google Scholar] [CrossRef]
- Zhao, G.H.; Zhang, P.; Li, J.; Zhang, Z.; Li, H.Y.; Ma, L.F. Effects of different scannin speeds on microstructure evolution and tribological properties of Inconel 718 alloy vacuumelectron beam surface modification. Mater. Today Sustain. 2024, 25, 100613. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhang, H.; Xin, Z.; Guo, J.; Dong, Y. Study on micro—Melt polishing and strengthening mechanism of scanning electron beam surface. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Withmaterials At. 2021, 504, 58–63. [Google Scholar] [CrossRef]











| Element | C% | Si% | Mn% | S% | P% | Cr% | Mo% | Ni% | Cu% |
|---|---|---|---|---|---|---|---|---|---|
| Technical requirement | 0.17~0.24 | 0.17~0.34 | 0.40~0.70 | ≤0.035 | ≤0.035 | 0.80~1.10 | 0.15~0.25 | ≤0.3 | ≤0.3 |
| Analysis results | 0.22 | 0.24 | 0.45 | 0.020 | 0.017 | 0.95 | 0.18 | 0.17 | 0.23 |
| Name | Preoxidation | Heat | Carburization | Diffuse | Heat Preservation | Quenching | Cleanse | Tempering |
|---|---|---|---|---|---|---|---|---|
| Station number | 4 | 6 | 28 | 7 | 1 | 1 | 3 | 9 |
| Temperature (°C) | 450 | 920 ± 10 | 920 ± 10 | 50 ± 10 | 850 ± 10 | 60–90 | 50 | 190 ± 10 |
| Carbon potential (%) | / | / | 1.1 | 0.8 | 0.8 | / | / | / |
| Time (min) | 240 | 360 | 480 | 420 | ≤60 | ≤60 | ≤180 | 240 |
| Gas | Air | N2-CH4O | N2,CH4O, Natural gas | N2 | Protective gas | Air | Air | |
| Current (mA) | Speed (mm/min) | Radius (mm) |
|---|---|---|
| 3 | 250 | 5 |
| 3 | 300 | 5 |
| 3 | 350 | 5 |
| 4 | 250 | 5 |
| 4 | 300 | 5 |
| 4 | 350 | 5 |
| 5 | 250 | 5 |
| 5 | 300 | 5 |
| 5 | 350 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Qu, J.-J.; Liu, F.; Yue, G.; Zhou, L.; Luo, Y.-C.; Ning, H.-W. Effect of Scanning Electron Beam Pretreatment on Gas Carburization of 22CrMoH Gear Steel. Coatings 2024, 14, 611. https://doi.org/10.3390/coatings14050611
Jiang W, Qu J-J, Liu F, Yue G, Zhou L, Luo Y-C, Ning H-W. Effect of Scanning Electron Beam Pretreatment on Gas Carburization of 22CrMoH Gear Steel. Coatings. 2024; 14(5):611. https://doi.org/10.3390/coatings14050611
Chicago/Turabian StyleJiang, Wei, Jing-Jing Qu, Fei Liu, Gao Yue, Lei Zhou, Yu-Cheng Luo, and Hui-Wang Ning. 2024. "Effect of Scanning Electron Beam Pretreatment on Gas Carburization of 22CrMoH Gear Steel" Coatings 14, no. 5: 611. https://doi.org/10.3390/coatings14050611




