Widening Genetic Diversity Using Embryo Rescue in Cucurbit Crops: A Review
Abstract
:1. Introduction
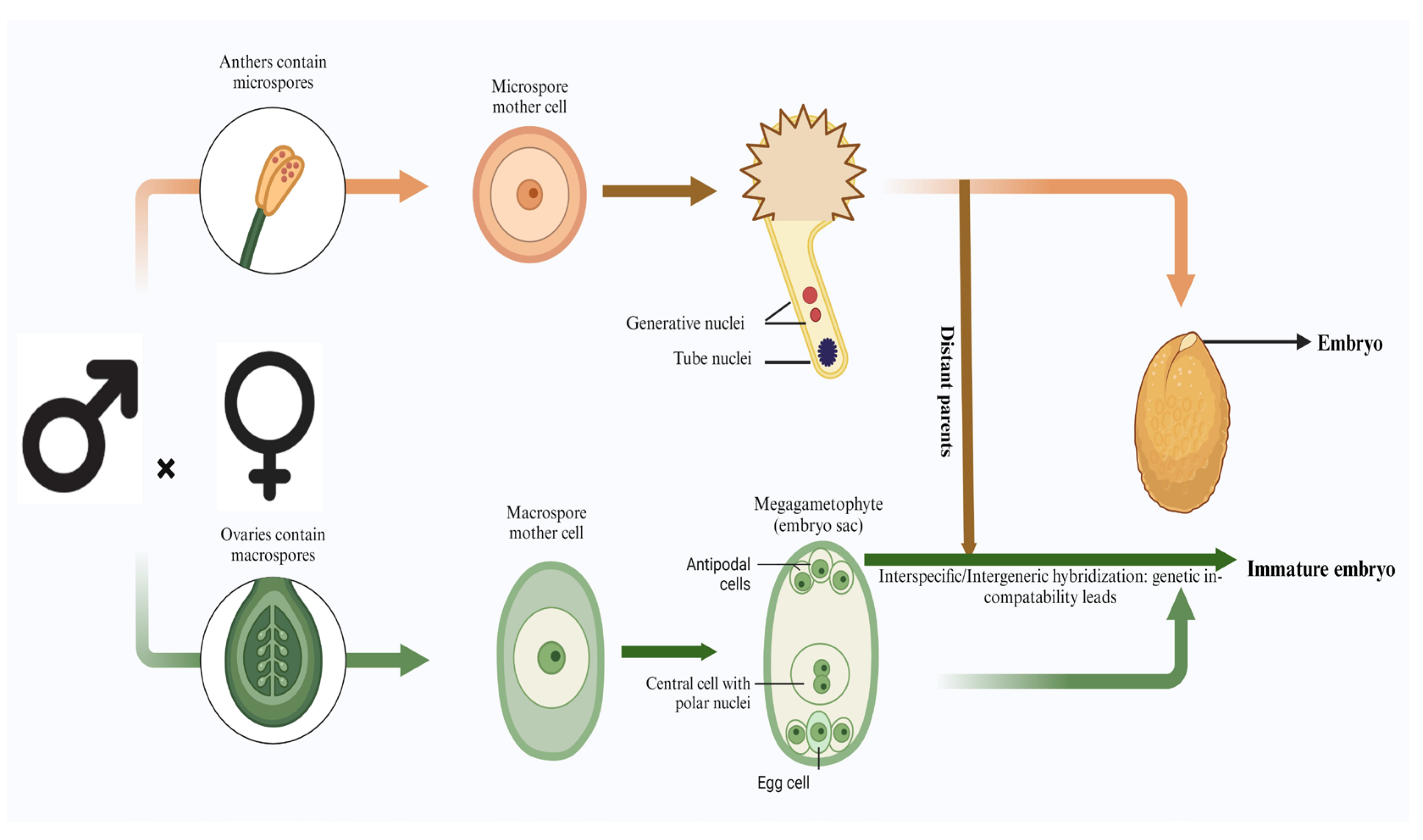
2. Factors Leading to Immature Embryos in Plant Breeding
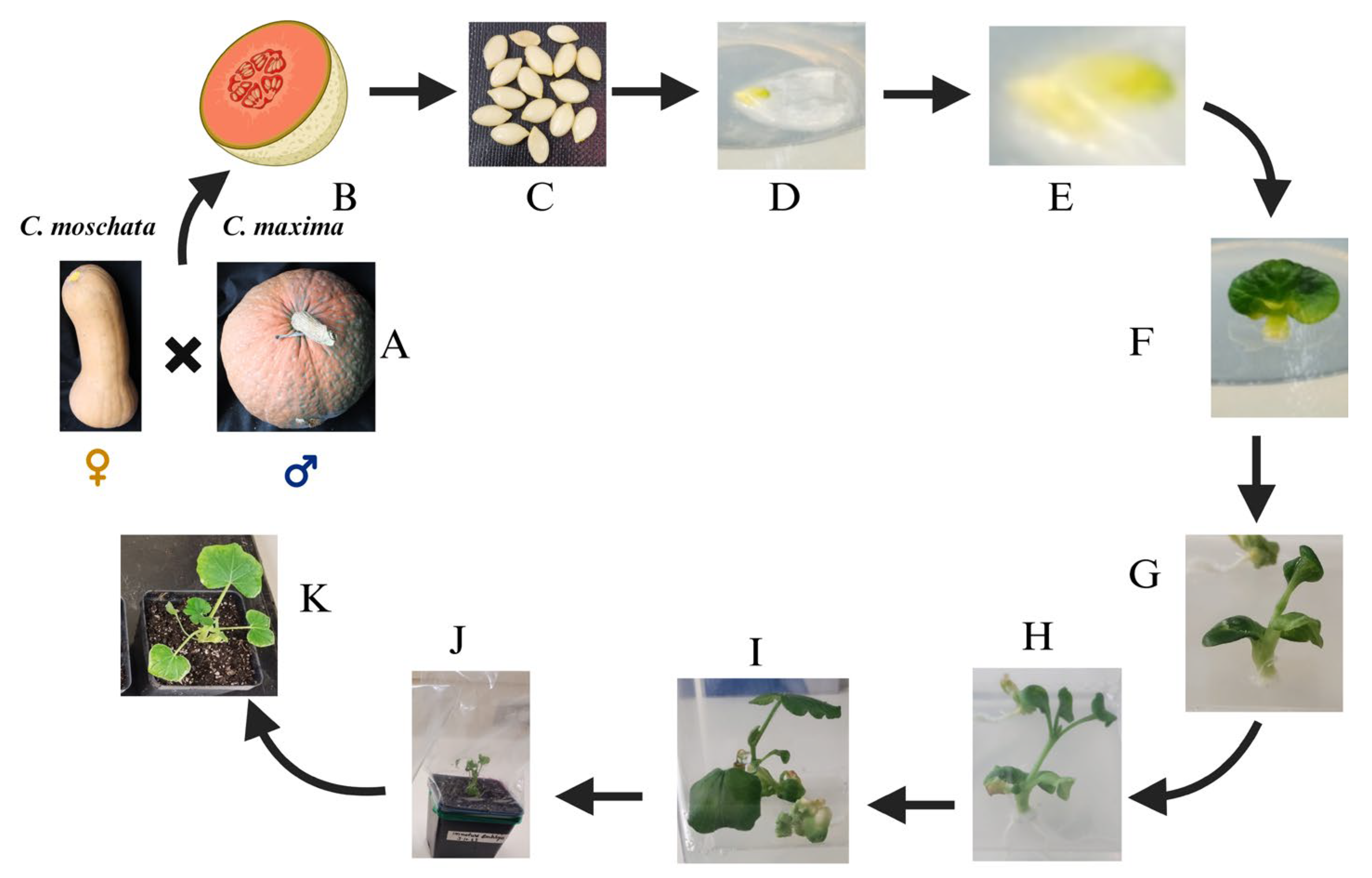
3. Historical Background of Embryo Rescue
4. Effect of Media Composition on Embryo Rescue
4.1. Interspecific/Intergeneric Hybridization
4.2. Haploidization
4.3. Seed Dormancy Breaking
5. Other Factors Influencing Embryo Rescue
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J. Ethnopharmacol. 2022, 282, 114599. [Google Scholar] [CrossRef]
- Parvathi, M.S.; Antony, P.D.; Kutty, M.S. Multiple stressors in vegetable production: Insights for trait-based crop improvement in cucurbits. Front. Plant Sci. 2022, 13, 861637. [Google Scholar] [CrossRef] [PubMed]
- Lebeda, A.; Widrlechner, M.P.; Staub, J.; Ezura, H.; Zalapa, J. Cucurbits (Cucurbitaceae; Cucumis spp., Cucurbita spp., Citrullus spp.), genetic resources, chromosome engineering and crop improvement. NCRPIS Publ. Pap. 2007, 8, 271–376. [Google Scholar]
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.; Mazourek, M.; Reitsma, K.; Fei, Z. Genetic resources and vulnerabilities of major cucurbit crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype stability and unbiased fractionation in the paleo-allotetraploid Cucurbita genomes. Mol. Plant. 2017, 10, 1293–1306. [Google Scholar] [CrossRef]
- Ranjan, J.K.; Pandey, S.; Prgaya Akhter Ansari, W.; Krishna, R.; Tarique Zeyad, M.; Singh, V. Biotic Stresses in Cucurbits: Status, Challenges, Breeding and Genetic Tools to Enhance Resistance. In Genomic Designing for Biotic Stress Resistant Vegetable Crops; Springer: Cham, Switzerland, 2022; pp. 345–379. [Google Scholar]
- Xanthopoulou, A.; Paris, H.S.; Tsompanoglou, I.; Polidoros, A.N.; Mellidou, I.; Ganopoulos, I. Genomic designing for abiotic stress tolerance in cucurbits. In Genomic Designing for Abiotic Stress Resistant Vegetable Crops; Springer: Cham, Switzerland, 2022; pp. 187–252. [Google Scholar]
- Pramanik, K.; Sahoo, J.P.; Mohapatra, P.P.; Acharya, L.K.; Jena, C. Insights into the embryo rescue—A modern in-vitro crop improvement approach in horticulture. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 20–33. [Google Scholar]
- Amiteye, S. In Vitro Embryo Rescue Techniques and Applications in Hybrid Plant Development. In Advanced Crop Improvement, Volume 2: Case Studies of Economically Important Crops; Springer: Cham, Switzerland, 2023; pp. 419–456. [Google Scholar]
- Bhattarai, S.P.; de la Pena, R.C.; Midmore, D.J.; Palchamy, K. In vitro culture of immature seed for rapid generation advancement in tomato. Euphytica 2009, 167, 23–30. [Google Scholar] [CrossRef]
- Shen, X.; Gmitter, F.G.; Grosser, J.W. Immature embryo rescue and culture. In Plant Embryo Culture: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 75–92. [Google Scholar]
- Bermejo, C.; Gatti, I.; Cointry, E. In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. 2016, 127, 585–590. [Google Scholar] [CrossRef]
- Sahijram, L.; Madhusudhana Rao, B. Hybrid embryo rescue in crop improvement. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 363–384. [Google Scholar]
- Kausch, A.P.; Tilelli, M.; Hague, J.; Heffelfinger, C.; Cunha, D.; Moreno, M.; Nelson, K. In situ embryo rescue for generation of wide intra- and interspecific hybrids of Panicum virgatum L. Plant Biotechnol. J. 2016, 14, 2168–2175. [Google Scholar] [CrossRef]
- Krishna, R.; Khandagale, K.; Benke, A.P.; Soumia, P.S.; Manjunathagowda, D.C.; Ansari, W.A.; Singh, M. Embryo rescue: A potential tool for improvement of economically important crops. In Advances in Plant Tissue Culture; Academic Press: Cambridge, MA, USA, 2022; pp. 259–282. [Google Scholar]
- Rogo, U.; Fambrini, M.; Pugliesi, C. Embryo Rescue in Plant Breeding. Plants 2023, 12, 3106. [Google Scholar] [CrossRef] [PubMed]
- Metwally, E.I.; Haroun, S.A.; El-Fadly, G.A. Interspecific cross between Cucurbita pepo L. and Cucurbita martinezii through in vitro embryo culture. Euphytica 1996, 90, 1–7. [Google Scholar] [CrossRef]
- de Oliveira, A.C.B.; Maluf, W.R.; Pinto, J.E.B.; Azevedo, S.M. Resistance to papaya ringspot virus in summer squash Cucurbita pepo L. introgressed from an interspecific C. pepo × C. moschata cross. Euphytica 2003, 132, 211–215. [Google Scholar] [CrossRef]
- Nuñez-Palenius, H.G.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Muskmelon embryo rescue techniques using in vitro embryo culture. In Plant Embryo Culture: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–115. [Google Scholar]
- Kaur, B.; Garcha, K.S.; Sandhu, J.S.; Sharma, M.; Dhatt, A.S. Interspecific hybridization for transfer of hull-less seed trait from Cucurbita pepo to C. moschata. Sci. Rep. 2023, 13, 4627. [Google Scholar] [CrossRef] [PubMed]
- Rakha, M.T.; Metwally, E.I.; Moustafa, S.A.; Etman, A.A.; Dewir, Y.H. Production of Cucurbita interspecific hybrids through cross pollination and embryo rescue technique. World Appl. Sci. J. 2012, 20, 1366–1370. [Google Scholar]
- Lotfi, M.; Salehi, S.; Pitrat, M. Detection of cucumber parthenogenic haploid embryos by floating of immature seeds in liquid medium. In Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008. [Google Scholar]
- Moon, P.; Meru, G. Embryo rescue of aged Cucurbita pepo seeds using squash rescue medium. J. Hortic. Sci. Res. 2018, 2, 62–69. [Google Scholar]
- Fu, Y.; Shrestha, S.; Moon, P.; Meru, G. Embryo Rescue Protocol for Interspecific Hybridization in Squash. JoVE J. Vis. Exp. 2022, 187, e64071. [Google Scholar]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.; Tester, M.; Wulff, B.B. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Claveria, E.; Garcia-Mas, J.; Dolcet-Sanjuan, R. Optimization of cucumber doubled haploid line production using in vitro rescue of in vivo induced parthenogenic embryos. J. Am. Soc. Horti Sci. 2005, 130, 555–560. [Google Scholar] [CrossRef]
- Diao, W.P.; Jia, Y.Y.; Song, H.; Zhang, X.Q.; Lou, Q.F.; Chen, J.F. Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenerates using SSR markers. Sci. Hortic. 2009, 119, 246–251. [Google Scholar] [CrossRef]
- Zohura, F.T.; Haque, M.E.; Islam, M.A.; Khalekuzzaman, M.; Sikdar, B. Establishment of an efficient in vitro regeneration system of ridge gourd (Luffa acutangula L. Roxb) from immature embryo and cotyledon explants. Intl. J. Sci. Technol. Res. 2013, 2, 33–37. [Google Scholar]
- Dhumal, S.S.; Naik, B.V.; Nimbalkar, M.S. Advances in tissue culture of cucurbits: A Review. Int. J. Curr. Microb. Appl. Sci. 2020, 9, 2887–2910. [Google Scholar] [CrossRef]
- Armenta-Medina, A.; Gillmor, C.S.; Gao, P.; Mora-Macias, J.; Kochian, L.V.; Xiang, D.; Datla, R. Developmental and genomic architecture of plant embryogenesis: From model plant to crops. Plant Commun. 2021, 2, 100136. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; Nijs, T.P.M.; Peloquin, S.J.; Hanneman, R.E. The significance of genic balance to endosperm development in interspecific crosses. Theor. Appl. Genet. 1980, 57, 5–9. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yabuno, T. Causal relationships between the polar nuclei in double fertilization and interspecific cross-incompatibility in Avena. Cytologia 1978, 43, 453–466. [Google Scholar] [CrossRef]
- Dilkes, B.P.; Comai, L. A differential dosage hypothesis for parental effects in seed development. Plant Cell 2004, 16, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Palenius, H.G.; Gomez-Lim, M.; Ochoa-Alejo, N.; Grumet, R.; Lester, G.; Cantliffe, D.J. Melon fruits: Genetic diversity, physiology, and biotechnology features. Crit. Rev. Biotechnol. 2008, 28, 13–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo rescue in plants: A review. Euphytica 1996, 89, 325–337. [Google Scholar] [CrossRef]
- Hanning, E. Zur physiologie pflanzlicher embryonen. I. Ueber die cultur von cruciferen-embryonen ausserhalb des embrysacks. Bot. Ztg. 1904, 62, 45–80. [Google Scholar]
- Raghavan, V. One hundred years of zygotic embryo culture investigations. Vitr. Cell Dev. Biol. Plant. 2003, 39, 437–442. [Google Scholar] [CrossRef]
- Brown, H.T. On the culture of the exised embryos of barley on nutrient solutions containing nitrogen in different forms. Trans. Guinness Res. Lab. 1906, 1, 288–299. [Google Scholar]
- Dubard, M.; Urbain, J.-A. De l’influence de l’albumen sur le développment de l’embryonen. Compt. Rend. Acad. Sci. 1913, 156, 1086–1089. [Google Scholar]
- Buckner, G.D.; Kastle, J.H. The growth of isolated plant embryos. J. Biol. Chem. 1917, 29, 209–213. [Google Scholar] [CrossRef]
- Andronescu, D.I. Germination and further development of the embryo of Zea mays separated from the endosperm. Am. J. Bot. 1919, 6, 443–452. [Google Scholar] [CrossRef]
- Dieterich, K. Über kultur von embryonen außerhalb des samens. Flora 1924, 117, 379–417. [Google Scholar] [CrossRef]
- Laibach, F. Das taubwerden von bastardsamen und die künstliche Aufzucht früh absterbender bastardembryonen. Z. Bot. 1925, 17, 417–459. [Google Scholar]
- Chen, J.F.; Staub, J.E.; Tashiro, Y. Regeneration of interspecific hybrids of Cucumis sativus L. × C. hystrix Char by direct embryo culture. Cucurbit Genet. Coop. Rpt. 1996, 19, 34–35. [Google Scholar]
- Šiško, M.; Ivančič, A.; Bohanec, B. Genome size analysis in the genus Cucurbita and its use for determination of interspecific hybrids obtained using the embryo-rescue technique. Plant Sci. 2003, 165, 663–669. [Google Scholar] [CrossRef]
- Skálová, D.; Dziechciarková, M.; Lebeda, A.; Křístková, E.; Navrátilová, B. Interspecific hybridization of Cucumis anguria and C. zeyheri via embryo-rescue. Biol. Plant. 2008, 52, 775–778. [Google Scholar] [CrossRef]
- Custers, J.B.M.; Bergervoet, J.H.W. In vitro culture of embryos of Cucumis spp.: Heart-stage embryos have a higher ability of direct plant formation than advanced-stage embryos. Sex. Plant Reprod. 1990, 3, 152–159. [Google Scholar] [CrossRef]
- Sauton, A.; Vaulx, R.D. Obtention de plantes haploïdes chez le melon (Cucumis melo L.) par gynogenèse induite par du pollen irradié. Agronomie 1987, 7, 141–148. [Google Scholar] [CrossRef]
- Sauton, A. Effect of season and genotype on gynogenetic haploid production in muskemlon, Cucumis melo L. Sci. Hortic. 1988, 35, 71–75. [Google Scholar] [CrossRef]
- Sauton, A. Haploid gynogenesis in Cucumis sativus induced by irradiated pollen. Cucurbit Genet. Coop 1989, 12, 22–23. [Google Scholar]
- Katarzyne, N.-S.; de Vaulx, R.D. Preliminary data on haploid cucumber (Cucumis sativus L.) induction. Cucurbit Genet Coop 1989, 12, 24–25. [Google Scholar]
- Yetisir, H.; Sari, N. A new method for haploid muskmelon (Cucumis melo L.) dihaploidization. Sci. Hortic. 2003, 98, 277–283. [Google Scholar] [CrossRef]
- Lotfi, M.; Kashi, A.; Onsinejad, R. Induction of parthenogenetic embryos by irradiated pollen in cucumber. Int. Symp. Cucurbits 1997, 492, 323–328. [Google Scholar] [CrossRef]
- Faris, N.M.; Nikolova, V.; Niemirowicz-Szczytt, K. The effect of gamma irradiation dose on cucumber (Cucumis sativus L.) haploid embryo production. Acta Physiol Plant 1991, 21, 391–396. [Google Scholar] [CrossRef]
- Lotfi, M.; Alan, A.R.; Henning, M.J.; Jahn, M.M.; Earle, E.D. Production of haploid and doubled haploid plants of melon (Cucumis melo L.) for use in breeding for multiple virus resistance. Plant Cell Rep. 2003, 21, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Stzangret, J.; Wronka, J.; Galecka, T.; Korzeniewska, A.; Niemirowicz-Szczytt, K. Cucumber (Cucumis sativus L.) haploids developed from parthenocarpic hybrids. In Progress in Cucurbit Genetics and Breeding Research, Proceedings of Cucurbitaceae; EUCARPIA: Wageningen, The Netherlands, 2004; pp. 411–414. [Google Scholar]
- Ari, E.; Ikten, H.; Gocmen, M.; Coskun, R.; Eren, A. Comparative evaluation of different embryo rescue techniques on parthenogenetic melon (Cucumis melo L.) fruits induced with irradiated pollen. Afr. J. Biotechnol. 2010, 9, 5347. [Google Scholar]
- Kurtar, E.S.; Balkaya, A.; Ozbakir, M.; Ofluoglu, T. Induction of haploid embryo and plant regeneration via irradiated pollen technique in pumpkin (Cucurbita moschata Duchesne ex. Poir). Afr. J. Biotechnol. 2009, 8, 5944–5951. [Google Scholar]
- Kurtar, E.S.; Balkaya, A. Production of in vitro haploid plants from in situ induced haploid embryos in winter squash (Cucurbita maxima Duchesne ex Lam.) via irradiated pollen. Plant Cell Tissue Organ Cult. 2010, 102, 267–277. [Google Scholar] [CrossRef]
- Godbole, M.; Murthy, H.N. Parthenogenetic haploid plants using gamma irradiated pollen in snapmelon (Cucumis melo var. momordica). Plant Cell Tiss Organ Cult. 2012, 109, 167–170. [Google Scholar] [CrossRef]
- Baktemur, G.; Taşkın, H.; Büyükalaca, S. Comparison of different methods for separation of haploid embryo induced through irradiated pollen and their economic analysis in melon (Cucumis melo var. inodorus). Sci. World J. 2013, 2013, 529502. [Google Scholar] [CrossRef]
- Gok Guler, P.; Sari, N.; Yetisir, H.; Yegul, M.; Kantoglu, Y.; Kunter, B. The effects of genotypes and irradiation doses on haploid embryo induction and plant production in bottle gourd [Lagenaria siceraria (Malign) Stanley]. In Proceedings of the V International Symposium on Cucurbits, Cartagena, Spain, 22–26 June 2015; Volume 1151, pp. 135–142. [Google Scholar]
- Kurtar, E.S.; Seymen, M.; Cetin, A.N.; Türkmen, O. Dihaploidization in Promising Summer Squash Genotypes (Cucurbita pepo L.) via Irradiated Pollen Technique. Yuz. Yıl Univ. J. Agric. Sci. 2021, 31, 42–51. [Google Scholar] [CrossRef]
- Bagheri, L.; Lotfi, M.; Nori, M. Production of haploid embryos and plants in Iranian melon (Cucumis melo L.) through irradiated pollen-induced parthenogenesis. In Mutation Breeding, Genetic Diversity and Crop Adaptation to Climate Change; CABI: Wallingford, UK, 2021; pp. 127–133. [Google Scholar]
- Hooghvorst, I.; Torrico, O.; Hooghvorst, S.; Nogués, S. In situ parthenogenetic doubled haploid production in melon “Piel de Sapo” for breeding purposes. Front. Plant Sci. 2020, 11, 522628. [Google Scholar] [CrossRef]
- Nunez-Palenius, H.G.; Klee, H.J.; Cantliffe, D.J. Embryo-rescue culture of the ‘Galia’ muskmelon (Cucumis melo L. var. reticulatus Ser.) male parental line. Plant Cell Tissue Organ Cult. 2006, 85, 345–352. [Google Scholar] [CrossRef]
- Hazem, F.; Golabadi, M. Use of chemical mutagens for production of inactive pollen grains, embryo rescue, and morphological changes in cucumber. Turk. J. Agric. For. 2018, 42, 11–21. [Google Scholar] [CrossRef]
- Ezura, H.; Akasaka-Kennedy, Y. Somatic embriogenesis in model cultivar, PI 161375 (Cucumis melo subsp. agrestis), of melon. In Progress in Cucurbit Genetics and Breeding Research. Proceedings of Cucurbitaceae; EUCARPIA: Wageningen, The Netherlands, 2004; pp. 431–435. [Google Scholar]
- De Jeu, M.J. In vitro techniques for ornamental breeding. Acta Hortic. 2000, 508, 55–60. [Google Scholar] [CrossRef]
- Moyle, L.C.; Jewell, C.P.; Kostyun, J.L. Fertile approaches to dissecting mechanisms of premating and postmating prezygotic reproductive isolation. Curr. Opin. Plant Biol. 2014, 18, 16–23. [Google Scholar] [CrossRef]
- Raghavan, V. Experimental Embryogenesis in Vascular Plants; Experimental Botany; Academic Press: London, UK, 1976; Volume 10, pp. 187–192. [Google Scholar]
- Pierik, R.L.M. In Vitro Culture of Higher Plants; Martinus Nijhoff Publisher: Dordrecht, The Netherlands, 1987; p. p. 344. ISBN 9789024735310. [Google Scholar]
- Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue culture in ornamentals: Cultivation factors, propagation techniques, and its application. Plants 2022, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- Zenkteler, M.E.; Nitzsche, W. In vitro culture of ovules of Triticum aestivum at early stages of embryogenesis. Plant Cell Rep. 1985, 4, 168–171.Ha. [Google Scholar] [CrossRef] [PubMed]

| S No | Crop 1 | Crop 2 | Application/Trait | Medium Used | Used Hormones | Reference |
|---|---|---|---|---|---|---|
| 1. | C. ficifolia, C. martinezii | C. pepo (Queen F1), C. pepo (MHTC77 F 1) | Hybridization | MS medium | MS medium supplemented with 0.1 mg/L kinetin and 0.01 mg/L indol acetic acid (IAA) | [22] |
| 2. | C. maxima, C. pepo, C. ficifolia, C. maxima, C. argyrosperma | C. pepo, C. moschata, C. maxima | Hybridization | MS basal medium | 0.1 mg/L kinetin, 0.01 mg/L indole-3-acetic acid and sucrose increased to 20 g/L | [47] |
| 3. | C. pepo | C. moschata | Hybridization | MS medium | 0.1 mg/Lkinetin, 0.01 mg/L indole-3-acetic acid | [25] |
| 4. | Cucumis anguria | C. zeyheri | Hybridization |
| BAP; 0.01 mg dm−3 IBA; 0.01 mg dm−3, | [48] |
| 5. | C. melo | Hybridization | E21 Medium | Putriscine, glutamine, coconut water | [20] | |
| 7. | Wild Cucurbita martinezii Bailey (the male parent) | Domesticated C. pepo L. (the female parent | Hybridization | Murashige and Skoog medium | 0.01 mg/L IAA and 0.1 mg/L KIN | [18] |
| 8. | Cucurbita pepo L—squash | Germplasm rescue |
| It contains E20/21 major salts 5 mL/L, minor salts 0.1 mL/L, sucrose 12 g/mL, IAA and IBA 0.01 µg, respectively | [24] | |
| 9. | Cucumis sativus L. and wild species. | C. zeyheri 2 x Sond. and C. metuliferus Naud | Hybridization | MS medium. | 0.1 mg/L kinetin, 0.01 mg/L (IAA), and 3.5% sucrose | [49] |
| 10. | C. hystrix | C. sativus | Hybridization | MS medium | Hormone-free solid media with 3% sucrose P.H.6 | [46] |
| 11. | C. pepo | C. moschata | Hybridization (Hull-less seed production) | MS media | 0.01 IAA mg/L and 0.1 Kinetin mg/L | [21] |
| S No | Crop 1 | Crop 2 | Application/Trait | Medium Used | Used Hormones | Reference |
|---|---|---|---|---|---|---|
| 1. | Galia muskmelon male parental line | transgenic and wild type | Haploid production Developed male parental line | Basic E20 basic medium and E21A medium with six new supplements | 0.01 mg/L Indole acetic acid, 0.01 mg/L BAP, 5% coconut water | [68] |
| 2. | Cucumis sativus L. | Haploid production | E20H8 medium | E20 supplemented with 7.9 mM CaCl2·2H2O, 0.17 mM CoCl2·6H2O, 0.10 mM FeEDTA, 20 g/L sucrose, and 8 g/L Bacto agar. pH 5.9. | [28] | |
| 3. | Cucurbits | Haploid production | E20A MS medium | BAP + Kin + IBA 2 + 1 + 0.5) mg/L | [69] | |
| 4. | C. moschata | Duchesne ex. Poir—pumpkin | Haploid | E20A medium | No hormones used | [60] |
| 5. | Melon genotypes Y2 and Y3 | Haploid production | CP nutrient media, E20A medium | vitamin B12, 0.02 mg/L IAA | [63] | |
| 6. | Winter squash (C. maxima Duchesne ex Lam.) | Haploid production | E20A medium | Along with media 0.01 mg/L IAA | [61] | |
| 7. | Cucumber | (Pol10, Rubin, Stawko) | Haploid production | E20A medium | Modified MS media | [58] |
| 8. | Snapmelon (Cucumis melo var. momordica) | Haploid production | E20A medium | Supplemented with 2% sucrose and 0.06 µM IAA | [62] | |
| 9. | Iranian melon cultivars | Haploid production. | E20A medium. | Embryo rescue was used with three methods: direct, liquid, and integrated. | [66] | |
| 10 | Cucumis sativus L. | Haploid production | E20A medium | [56] | ||
| 11. | Cucumber | Genotype soltar and monarch | Haploid production | E20A liquid medium | Solid medium also | [23] |
| 12. | C. melo | Hapolid production | MS media and E20A medium | MS medium with 0.01% IAA | [59] | |
| 13. | Summer squash (C. pepo) | 14 varieties used | Dihaplodization. | Modified E20A medium | Only media | [65] |
| 14. | C. melo L. | Haploid and double haploid production against multiple virus resistance | E20A medium | Initially liquid media and further solid media | [57] | |
| 15. | Lagenaria siceraria (Malign) Stanley | Haploid embryo production | E20A medium | E20A Liquid medium | [64] | |
| 16. | Cucumber | Daminus) and two greenhouse cultivars (Rubah, RZ | Haploidization | E20A medium | No hormones used | [55] |
| 17. | PI 161375 (Cucumis melo L. subsp. agrestis, chinensis group) | Vedrantais (C. melo L. subsp. melo, cantalupensis group | Somatic embryogenesis | Embryo-induction (EI) medium | 0.1 mg/L BA and 2 mg/L 2,4-D | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, C.S.; Ramireddy, S.; Reddy, U.K. Widening Genetic Diversity Using Embryo Rescue in Cucurbit Crops: A Review. Plants 2024, 13, 1320. https://doi.org/10.3390/plants13101320
Reddy CS, Ramireddy S, Reddy UK. Widening Genetic Diversity Using Embryo Rescue in Cucurbit Crops: A Review. Plants. 2024; 13(10):1320. https://doi.org/10.3390/plants13101320
Chicago/Turabian StyleReddy, Chinreddy Subramanyam, Sahithi Ramireddy, and Umesh K. Reddy. 2024. "Widening Genetic Diversity Using Embryo Rescue in Cucurbit Crops: A Review" Plants 13, no. 10: 1320. https://doi.org/10.3390/plants13101320





