Assessment of Anticancer Properties of Argemone mexicana L. and Berberine: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Lines
Ethics
2.3. Plant Material and Extraction
2.3.1. Extraction
2.3.2. Phytochemical Analysis
A. HPLC-MS
B. NMR
2.4. Cell Viability Assays
2.5. Hemolytic and Anti-Hemolytic Activity
2.5.1. Hemolytic Test
2.5.2. Anti-Hemolytic Test by the AAPH Assay
2.6. Lethality in Artemia salina
2.7. Antioxidant Activities
2.7.1. DPPH Scavenging Test
2.7.2. ABTS Scavenging Test
2.7.3. FRAP Scavenging Test
2.8. Nitric Oxide Production
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Data of Argemone mexicana
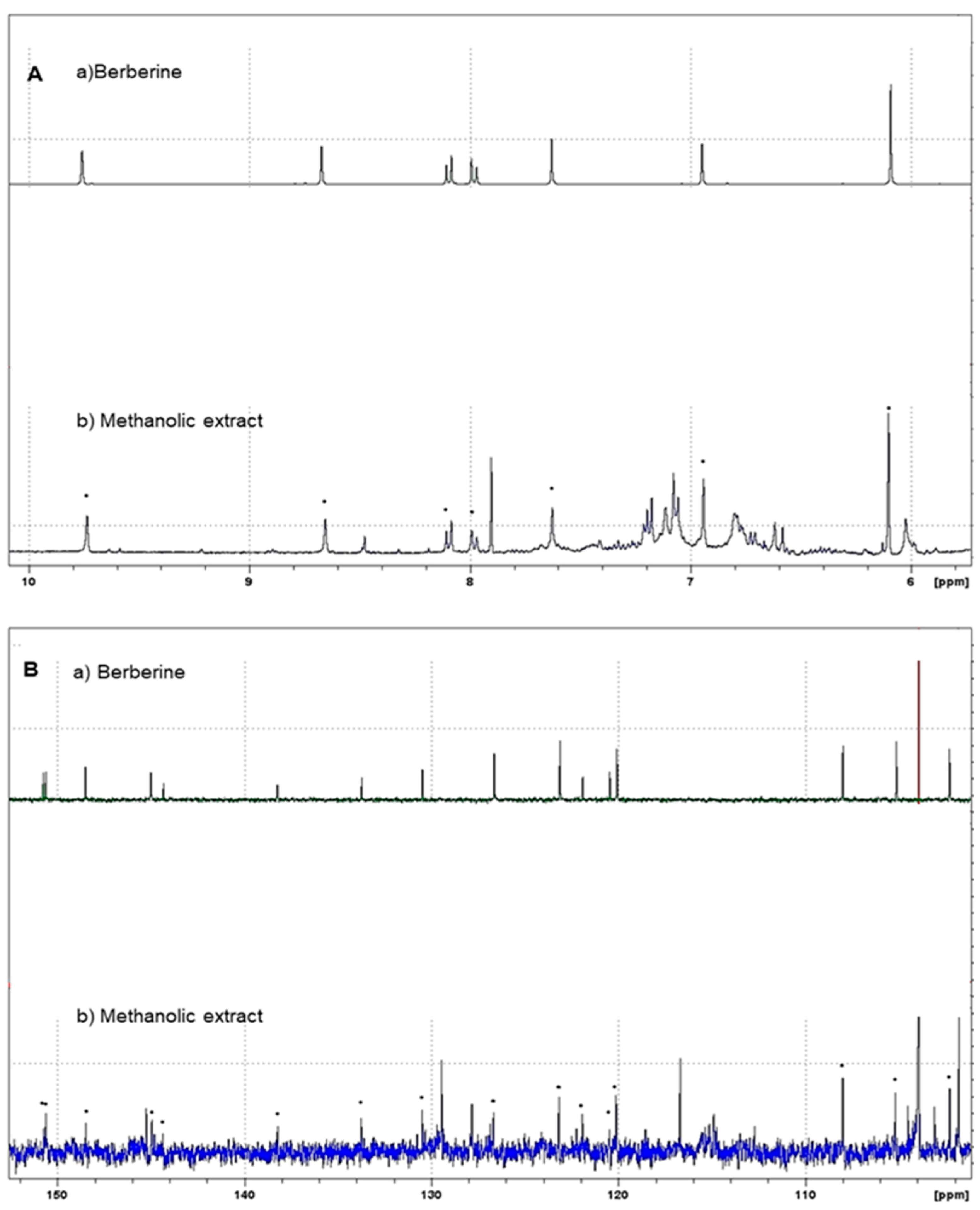
H-NMR and 13C-NMR
3.2. Cytotoxic Activity and SI
3.3. Hemolytic and Anti-hemolytic Activity
3.4. Effect on A. salina and Antioxidant Activity
3.5. NO Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tienda-Vázquez, M.A.; Melchor-Martínez, E.M.; Elizondo-Luévano, J.H.; Parra-Saldívar, R.; Lara-Ortiz, J.S.; Luna-Sosa, B.; Scheckhuber, C.Q. Antidiabetic Plants for the Treatment of Type 2 Diabetes Mellitus and Associated Bacterial Infections. Processes 2023, 11, 1299. [Google Scholar] [CrossRef]
- De La Cruz-Jiménez, L.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- González-Meza, G.M.; Elizondo-Luevano, J.H.; Cuellar-Bermudez, S.P.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. New Perspective for Macroalgae-Based Animal Feeding in the Context of Challenging Sustainable Food Production. Plants 2023, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Issa, K.; Bakhatan, A.; Khaled, M.A.; Jaradat, N.; Hawash, M.; Al-Maharik, N.; Ghanim, M.; Qadi, M. Exploring the Phytoconstituents, Antimicrobial Potency, and Cytotoxic Effects of Essential Oil from Origanum punonense from Palestine. BMC Complement. Med. Ther. 2024, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Sam, S. Importance and Effectiveness of Herbal Medicines. J. Pharmacogn. Phytochem. 2019, 8, 354–357. [Google Scholar]
- Kumari, R.; Kotecha, M. A Review on the Standardization of Herbal Medicines. Int. J. Pharma Sci. Res. 2016, 7, 97–106. [Google Scholar]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Vukić, M.D.; Čmiková, N.; Hsouna, A.B.; Saad, R.B.; Garzoli, S.; Schwarzová, M.; Vuković, N.L.; Obradović, A.D.; Matić, M.M.; Waszkiewicz-Robak, B.; et al. Thymus Zygis, Valuable Antimicrobial (In Vitro and In Situ) and Antibiofilm Agent with Potential Antiproliferative Effects. Plants 2023, 12, 3920. [Google Scholar] [CrossRef] [PubMed]
- López-Villarreal, S.M.; Elizondo-Luévano, J.H.; Pérez-Hernández, R.A.; Sánchez-García, E.; Verde-Star, M.J.; Castro-Ríos, R.; Garza-Tapia, M.; Rodríguez-Luis, O.E.; Chávez-Montes, A. Preliminary Study of the Antimicrobial, Anticoagulant, Antioxidant, Cytotoxic, and Anti-Inflammatory Activity of Five Selected Plants with Therapeutic Application in Dentistry. Int. J. Env. Res. Public. Health 2022, 19, 7927. [Google Scholar] [CrossRef]
- Cárdenas Garza, G.R.; Elizondo Luévano, J.H.; Bazaldúa Rodríguez, A.F.; Chávez Montes, A.; Pérez Hernández, R.A.; Martínez Delgado, A.J.; López Villarreal, S.M.; Rodríguez Rodríguez, J.; Sánchez Casas, R.M.; Castillo Velázquez, U.; et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) Extracts for Their Applications as Natural Anti-Inflammatory Adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef]
- Ahmad Khan, M.S.; Ahmad, I. Herbal Medicine. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–13. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Mbaoji, F.N.; Onwuka, A.M.; Onu, S.; Peter, I.E.; Nweze, J.A.; Okonta, L.E. Evaluation of Methanol-Dichloromethane Extract of Stemonocoleus micranthus Harms (Fabaceae) Stem Bark for Anti-Inflammatory and Immunomodulatory Activities. Evid. Based Complement. Altern. Med. 2020, 2020, 1738163. [Google Scholar] [CrossRef]
- Schwarzbach, A.E.; Kadereit, J.W. Phylogeny of Prickly Poppies, Argemone (Papaveraceae), and the Evolution of Morphological and Alkaloid Characters Based on ITS NrDNA Sequence Variation. Plant Syst. Evol. 1999, 218, 257–279. [Google Scholar] [CrossRef]
- Rubio-Pina, J.; Vazquez-Flota, F. Pharmaceutical Applications of the Benzylisoquinoline Alkaloids from Argemone mexicana L. Curr. Top. Med. Chem. 2013, 13, 2200–2207. [Google Scholar] [CrossRef]
- Brahmachari, G.; Gorai, D.; Roy, R. Argemone mexicana: Chemical and Pharmacological Aspects. Rev. Bras. Farmacogn. 2013, 23, 559–575. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Garza-Vega, L.M.; Torres-Hernández, Á.D.; Quintanilla-Licea, R.; Chávez-Montes, A. Argemone mexicana (Papaveraceae) y Berberina—Tesoros Ocultos de La Medicina Herbal. Rev. De. Cienc. Agroaliment. Y Biotecnol. 2024, 1, 5–11. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, F.-R.; Khalil, A.T.; Hsieh, P.-W.; Wu, Y.-C. Cytotoxic Benzophenanthridine and Benzylisoquinoline Alkaloids from Argemone mexicana. Z. Naturforsch. C. J. Biosci. 2003, 58, 521–526. [Google Scholar] [CrossRef]
- Hussain, T.; Bajpai, S.; Saeed, M.; Moin, A.; Alafnan, A.; Khan, M.; Kamal, M.A.; Ganash, M.; Ashraf, G.M. Potentiating Effect of Ethnomedicinal Plants Against Proliferation on Different Cancer Cell Lines. Curr. Drug Metab. 2018, 19, 584–595. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, A.; Sánchez-García, E.; Quintanilla-Licea, R.; Bazaldúa-Rodríguez, A.F.; Pérez-Hernández, R.A.; Hernández-García, M.E.; Báez-González, J.G.; Castro-Ríos, R.; Elizondo-Luévano, J.H.; Chávez-Montes, A. Amoebicidal and Trichomonicidal Capacity of Polymeric Nanoparticles Loaded with Extracts of the Plants Curcuma longa (Zingiberaceae) and Berberis vulgaris (Berberidaceae). Rev. Biol. Trop. 2022, 70, 319–331. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Hernández-García, M.E.; Pérez-Narváez, O.A.; Castro-Ríos, R.; Chávez-Montes, A. Berberina, Curcumina y Quercetina Como Potenciales Agentes Con Capacidad Antiparasitaria. Rev. Biol. Trop. 2020, 68, 1241–1249. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Akhter, M.H.; Alam, M.S.; Ali, M.D.; Hussain, A. An Updated Review on Therapeutic Potential and Recent Advances in Drug Delivery of Berberine: Current Status and Future Prospect. Curr. Pharm. Biotechnol. 2022, 23, 60–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Piao, X.L.; Piao, X.S.; Lu, T.; Wang, D.; Kim, S.W. Preventive Effect of Coptis chinensis and Berberine on Intestinal Injury in Rats Challenged with Lipopolysaccharides. Food Chem. Toxicol. 2011, 49, 61–69. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology Effects of Berberis vulgaris (Barberry) and Its Active Constituent, Berberine: A Review. Iran. J. Basic. Med. Sci. 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Li, J.; Gu, L.; Zhang, H.; Liu, T.; Tian, D.; Zhou, M.; Zhou, S. Berberine Represses DAXX Gene Transcription and Induces Cancer Cell Apoptosis. Lab. Invest. 2013, 93, 354–364. [Google Scholar] [CrossRef]
- Guamán Ortiz, L.; Lombardi, P.; Tillhon, M.; Scovassi, A. Berberine, an Epiphany Against Cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New Perspectives for Old Remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Chávez-Montes, A.; Bazaldúa Rodríguez, A.F.; Larqué-García, H.; Gutiérrez-Soto, G.; Elizondo-Luévano, J.H. Actividad Antiparasitaria In-Vitro Del Extracto Metanólico de Kalanchoe daigremontiana (Crassulaceae) En Contra de Entamoeba histolytica (Amoebida: Entamoebidae) y Trichomonas vaginalis (Trichomonadida: Trichomonadidae). Sci. Agric. Vita 2024, 1, 1–9. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Biological Activity and Lymphoma Cell Growth Inhibition by Selected Mexican Medicinal Plants. Life 2023, 13, 958. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Gomez-Flores, R.; Verde-Star, M.J.; Tamez-Guerra, P.; Romo-Sáenz, C.I.; Chávez-Montes, A.; Rodríguez-Garza, N.E.; Quintanilla-Licea, R. In Vitro Cytotoxic Activity of Methanol Extracts of Selected Medicinal Plants Traditionally Used in Mexico against Human Hepatocellular Carcinoma. Plants 2022, 11, 2862. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana, NOM-253-SSA1-2012; Para la Disposición de Sangre Humana y Sus Somponentes con Fines Terapéuticos. Diario Oficial de la Federación: México 26 October 2012. Available online: http://www.cnts.salud.gob.mx/descargas/NOM-253-SSA1-2012.pdf (accessed on 11 May 2024).
- Rodríguez-Garza, N.E.; Molina-Garza, J.Z.; Galaviz-Silva, L.; Quintanilla-Licea, R. Evaluación In Vitro de Extractos de Plantas Medicinales Contra Trypanosoma cruzi, Agente Causal de La Enfermedad de Chagas. Rev. Tend. Docencia Investig. Química 2019, 5, 677–685. [Google Scholar]
- Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Verde-Star, M.J.; Hernández-García, M.E.; Vargas-Villarreal, J.; Garza-González, J.N. Furanocoumarins from Ruta chalepensis with Amebicide Activity. Molecules 2021, 26, 3684. [Google Scholar] [CrossRef]
- Hernández-Marín, D.A.; Guevara-Lara, F.; Rivas-Morales, C.; Verduzco-Martínez, J.A.; Galindo-Rodriguez, S.A.; Sánchez-García, E. Biological Activity of Nothoscordum bivalve (L.) Britton and Parthenium incanum Kunth Extracts. Indian. J. Tradit. Knowl. 2018, 17, 699–706. [Google Scholar]
- Elizondo-Luevano, J.H.; Verde-Star, J.; González-Horta, A.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In Vitro Effect of Methanolic Extract of Argemone mexicana against Trichomonas vaginalis. Korean J. Parasitol. 2020, 58, 135–145. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; Sánchez-García, E.; Hernández-García, M.E.; Vargas-Villarreal, J.; Rodríguez-Luis, O.E.; Chávez-Montes, A. In Vitro Study of Antiamoebic Activity of Methanol Extracts of Argemone mexicana on Trophozoites of Entamoeba histolytica HM1-IMSS. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7453787. [Google Scholar] [CrossRef]
- Orozco-Nunnelly, D.A.; Pruet, J.; Rios-Ibarra, C.P.; Bocangel Gamarra, E.L.; Lefeber, T.; Najdeska, T. Characterizing the Cytotoxic Effects and Several Antimicrobial Phytocompounds of Argemone mexicana. PLoS ONE 2021, 16, e0249704. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Rodríguez-Garza, N.E.; Bazaldúa-Rodríguez, A.F.; Romo-Sáenz, C.I.; Tamez-Guerra, P.; Verde-Star, M.J.; Gomez-Flores, R.; Quintanilla-Licea, R. Cytotoxic, Anti-Hemolytic, and Antioxidant Activities of Ruta chalepensis L. (Rutaceae) Extract, Fractions, and Isolated Compounds. Plants 2023, 12, 2203. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) Assay When Compared to Three Commonly Used Cell Enumeration Assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Shi, D.; Xu, W.; Wong, M.; Popovich, D.G. An Improved Purification Method for Removing Colour Interference from 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Antibacterial Assays. Appl. Sci. 2023, 13, 5067. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Rodríguez-Garza, N.E.; Torres-Hernández, Á.D.; Verde-Star, M.J.; Elizondo-Luévano, J.H. Actividad Citotóxica, Antioxidante y Antihemolítica Del Extracto Metanólico de Cymbopogon citratus (DC.) Stapf. Investig. Desarro. Cienc. Tecnol. Aliment. 2023, 8, 957–964. [Google Scholar] [CrossRef]
- Guillén-Meléndez, G.A.; Villa-Cedillo, S.A.; Pérez-Hernández, R.A.; Castillo-Velázquez, U.; Salas-Treviño, D.; Saucedo-Cárdenas, O.; Montes-de-Oca-Luna, R.; Gómez-Tristán, C.A.; Garza-Arredondo, A.J.; Zamora-Ávila, D.E.; et al. Cytotoxic Effect In Vitro of Acalypha monostachya Extracts over Human Tumor Cell Lines. Plants 2021, 10, 2326. [Google Scholar] [CrossRef]
- Cázares-Jaramillo, G.E.; Molina-Garza, Z.J.; Luna-Cruz, I.E.; Solís-Soto, L.Y.; Rosales-Encina, J.L.; Galaviz-Silva, L. In Vitro Anti-Trypanosoma cruzi Activity of Methanolic Extract of Bidens pilosa and Identification of Active Compounds by Gas Chromatography-Mass Spectrometry Analysis. Parasites Hosts Dis. 2023, 61, 405–417. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; Vicente, B.; Fernández-Soto, P.; López-Aban, J.; Muro, A.; Chávez-Montes, A. In Vitro Antischistosomal Activity of the Argemone mexicana Methanolic Extract and Its Main Component Berberine. Iran. J. Parasitol. 2021, 16, 91–100. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.A.; Leos-Rivas, C.; Oranday-Cárdenas, A.; Hernández-Luna, C.E.; Sánchez-García, E.; Rivas-Morales, C. Efecto In Vitro En La Inhibición Del Proceso de Nucleación En Litiasis Renal, Capacidad de Captura de Radicales Libres, Metanólico de Berberis trifoliata. Rev. Mex. Cienc. Farm. 2015, 46, 70–76. [Google Scholar]
- Pérez, K.C.; Galaviz, L.; Iracheta, J.M.; Lucero, E.A.; Molina, Z.J. Actividad Contra Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) de Extractos Metanólicos de Plantas de Uso Medicinal En México. Rev. Biol. Trop. 2017, 65, 1459–1469. [Google Scholar] [CrossRef]
- Liu, Y.; Long, S.; Zhang, S.; Tan, Y.; Wang, T.; Wu, Y.; Jiang, T.; Liu, X.; Peng, D.; Liu, Z. Synthesis and Antioxidant Activities of Berberine 9-: O -Benzoic Acid Derivatives. RSC Adv. 2021, 11, 17611–17621. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The Antioxidant and Antimicrobial Activity of Essential Oils against Pseudomonas spp. Isolated from Fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef]
- Huynh, T.T.H.; Wongmaneepratip, W.; Vangnai, K. Relationship between Flavonoid Chemical Structures and Their Antioxidant Capacity in Preventing Polycyclic Aromatic Hydrocarbons Formation in Heated Meat Model System. Foods 2024, 13, 1002. [Google Scholar] [CrossRef]
- Castillo-Velázquez, U.; Gomez-Flores, R.; Tamez-Guerra, R.; Tamez-Guerra, P.; Rodríguez-Padilla, C. Differential Responses of Macrophages from Bovines Naturally Resistant or Susceptible to Mycobacterium bovis after Classical and Alternative Activation. Vet. Immunol. Immunopathol. 2013, 154, 8–16. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; López-Abán, J.; Gorgojo-Galindo, O.; Fernández-Soto, P.; Vicente, B.; Muro, A.; Chávez-Montes, A. Berberine: A Nematocidal Alkaloid from Argemone mexicana against Strongyloides venezuelensis. Exp. Parasitol. 2021, 220, 108043. [Google Scholar] [CrossRef]
- Chávez Enciso, N.A.; Coy-Barrera, E.D.; Patiño, O.J.; Cuca, L.E.; Delgado, G. Evaluation of the Leishmanicidal Activity of Rutaceae and Lauraceae Ethanol Extracts on Golden Syrian Hamster (Mesocricetus auratus) Peritoneal Macrophages. Indian. J. Pharm. Sci. 2014, 76, 188–197. [Google Scholar]
- Lucía, C.-P.A.; Jacqueline, B.-R.; Alberto, B.-R.L.; David, B.-A.; Beatriz, R.-A. Actualized Inventory of Medicinal Plants Used in Traditional Medicine in Oaxaca, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Adil, M.; Filimban, F.Z.; Ambrin; Quddoos, A.; Sher, A.A.; Naseer, M. Phytochemical Screening, HPLC Analysis, Antimicrobial and Antioxidant Effect of Euphorbia parviflora L. (Euphorbiaceae Juss.). Sci. Rep. 2024, 14, 5627. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Peralta, A.A.; Robles-Zepeda, E.E.; Garibay-Escobar, A.; Ruiz-Bustos, E.; Alvarez-Berber, P.P.; Gálvez-Ruiz, C.C. In Vitro Anti-Proliferative Activity of Argemone gracilenta and Identification of Some Active Components. BMC Complement. Altern. Med. 2015, 15, 13. [Google Scholar] [CrossRef]
- Pan, J.-F.; Yu, C.; Zhu, D.-Y.; Zhang, H.; Zeng, J.-F.; Jiang, S.-H.; Ren, J.-Y. Identification of Three Sulfate-Conjugated Metabolites of Berberine Chloride in Healthy Volunteers’ Urine after Oral Administration. Acta Pharmacol. Sin. 2002, 23, 77–82. [Google Scholar] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Fan, Y. Antioxidant Activities of Berberine Hydrochloride. J. Med. Plants Res. 2011, 5, 3702–3707. [Google Scholar]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Mahmoudv, H.; Sharififar, F.; Sharifi, I.; Ezatpour, B.; Fasihi Harandi, M.; Makki, M.S.; Zia-Ali, N.; Jahanbakhsh, S. In Vitro Inhibitory Effect of Berberis vulgaris (Berberidaceae) and Its Main Component, Berberine against Different Leishmania Species. Iran. J. Parasitol. 2014, 9, 28–36. [Google Scholar]
- Mahmoudvand, H.; Ayatollahi Mousavi, S.A.; Sepahvand, A.; Sharififar, F.; Ezatpour, B.; Gorohi, F.; Saedi Dezaki, E.; Jahanbakhsh, S. Antifungal, Antileishmanial, and Cytotoxicity Activities of Various Extracts of Berberis vulgaris (Berberidaceae) and Its Active Principle Berberine. ISRN Pharmacol. 2014, 2014, 602436. [Google Scholar] [CrossRef]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic Effects of Bangladeshi Medicinal Plant Extracts. Evid. Based Complement. Altern. Med. 2011, 2011, 578092. [Google Scholar] [CrossRef]
- Radha Abbas Hasoon, M.; Jawad Kadhim, N. Improvement of the Selectivity Index (SI) and Cytotoxicity Activity of Doxorubicin Drug by Panax ginseng Plant Extract. Arch. Razi Inst. 2021, 76, 659–666. [Google Scholar] [CrossRef]
- Romero-Arguelles, R.; Romo-Sáenz, C.I.; Morán-Santibáñez, K.; Tamez-Guerra, P.; Quintanilla-Licea, R.; Orozco-Flores, A.A.; Ramírez-Villalobos, J.M.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Antitumor Activity of Endophytic and Rhizosphere Gram-Positive Bacteria from Ibervillea sonorae (S. Watson) Greene against L5178Y-R Lymphoma Cells. Int. J. Env. Res. Public. Health 2022, 19, 894. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Meléndez, G.A.; Soto-Domínguez, A.; de Loera-Arias, M.J.; Castillo-Velázquez, U.; Villa-Cedillo, S.A.; Piña-Mendoza, E.I.; Estrada-Castillón, E.; Chávez-Montes, A.; González-Alcocer, A.; Becerra-Verdín, E.M.; et al. Effect of Methanolic Extract of Mimosa malacophylla A.Gray in Vero and HEK-293 Cell Lines, and in the Morphology of Kidney and Bladder of Rats with Induced Urolithiasis. J. Ethnopharmacol. 2022, 297, 115552. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Oyenihi, O.; Rademan, S.; Erhabor, J.; Matsabisa, M.; Barker, J.; Langat, M.K.; Kendal-Smith, A.; Asemota, H.; Delgoda, R. Selective Cytotoxic and Anti-Metastatic Activity in DU-145 Prostate Cancer Cells Induced by Annona muricata L. Bark Extract and Phytochemical, Annonacin. BMC Complement. Med. Ther. 2020, 20, 375. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Durgawale, P.P.; Patil, M.N.; Hinge, D.D.; Jagdale, N.J.; Deshmukh, V.N.; More, A.L. Biogenic Synthesis of Gold Nanoparticles Using Argemone mexicana L. and Their Cytotoxic and Genotoxic Effects on Human Colon Cancer Cell Line (HCT-15). J. Genet. Eng. Biotechnol. 2021, 19, 9–11. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Wang, J.; Chen, H.; Tang, J.; Bian, A.-W.; Liu, T.; Yu, L.-F.; Yi, Z.; Yang, F. Synthesis and Anticancer Activity of Novel 9,13-Disubstituted Berberine Derivatives. Bioorg Med. Chem. Lett. 2020, 30, 126821. [Google Scholar] [CrossRef]
- Raghav, D.; Ashraf, S.M.; Mohan, L.; Rathinasamy, K. Berberine Induces Toxicity in HeLa Cells through Perturbation of Microtubule Polymerization by Binding to Tubulin at a Unique Site. Biochemistry 2017, 56, 2594–2611. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A. Mechanism of Action of Antitumor Drugs That Interact with Microtubules and Tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Downing, K.H. Structural Basis for the Interaction of Tubulin with Proteins and Drugs That Affect Microtubule Dynamics. Annu. Rev. Cell Dev. Biol. 2000, 16, 89–111. [Google Scholar] [CrossRef]
- Okon, E.; Luszczki, J.J.; Kukula-Koch, W.; Halasa, M.; Jarzab, A.; Khurelbat, D.; Stepulak, A.; Wawruszak, A. Synergistic or Additive Pharmacological Interactions between Magnoflorine and Cisplatin in Human Cancer Cells of Different Histological Origin. Int. J. Mol. Sci. 2020, 21, 2848. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Bobiński, M.; Okła, K.; Bednarek, W.; Wawruszak, A.; Dmoszyńska-Graniczka, M.; Garcia-Sanz, P.; Wertel, I.; Kotarski, J. The Effect of Fucoidan, a Potential New, Natural, Anti-Neoplastic Agent on Uterine Sarcomas and Carcinosarcoma Cell Lines: ENITEC Collaborative Study. Arch. Immunol. Ther. Exp. 2019, 67, 125–131. [Google Scholar] [CrossRef] [PubMed]
- More, N.; Kharat, A. Antifungal and Anticancer Potential of Argemone mexicana L. Medicines 2016, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.L.; Liu, Y.Q.; Wang, P.; Song, C.H.; Wang, K.J.; Dai, L.P.; Zhang, J.Y.; Ye, H. The Effect of Quercetin Nanoparticle on Cervical Cancer Progression by Inducing Apoptosis, Autophagy and Anti-Proliferation via JAK2 Suppression. Biomed. Pharmacother. 2016, 82, 595–605. [Google Scholar] [CrossRef]
- Niki, E. [3] Free Radical Initiators as Source of Water- or Lipid-Soluble Peroxyl Radicals. Methods Enzym. 1990, 186, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Antioxidative and Immunomodulating Properties of Aronia melanocarpa Extract Rich in Anthocyanins. Plants 2022, 11, 3333. [Google Scholar] [CrossRef] [PubMed]
- Nuruki, Y.; Matsumoto, H.; Tsukada, M.; Tsukahara, H.; Takajo, T.; Tsuchida, K.; Anzai, K. Method to Improve Azo-Compound (AAPH)-Induced Hemolysis of Erythrocytes for Assessing Antioxidant Activity of Lipophilic Compounds. Chem. Pharm. Bull. 2021, 69, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Racková, L.; Májeková, M.; Kost’álová, D.; Stefek, M. Antiradical and Antioxidant Activities of Alkaloids Isolated from Mahonia aquifolium. Structural Aspects. Bioorg Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef]
- Shou, J.-W.; Cheung, C.-K.; Gao, J.; Shi, W.-W.; Shaw, P.-C. Berberine Protects C17.2 Neural Stem Cells from Oxidative Damage Followed by Inducing Neuronal Differentiation. Front. Cell Neurosci. 2019, 13, 395. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Guzmán, L.; Jiménez-Aspee, F.; Echeverría, J.; Wehinger, S.; Valenzuela, C.; Araya-Maturana, R.; Martínez-Cifuentes, M. An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-Alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties. Antioxidants 2022, 11, 1104. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Álvarez-Alarcón, N.; Osorio-Méndez, J.J.; Ayala-Fajardo, A.; Garzón-Méndez, W.F.; Garavito-Aguilar, Z.V. Zebrafish and Artemia salina In Vivo Evaluation of the Recreational 25C-NBOMe Drug Demonstrates Its High Toxicity. Toxicol. Rep. 2021, 8, 315–323. [Google Scholar] [CrossRef]
- Colombo, M.L.; Bugatti, C.; Mossa, A.; Pescalli, N.; Piazzoni, L.; Pezzoni, G.; Menta, E.; Spinelli, S.; Johnson, F.; Gupta, R.C.; et al. Cytotoxicity Evaluation of Natural Coptisine and Synthesis of Coptisine from Berberine. Il Farm. 2001, 56, 403–409. [Google Scholar] [CrossRef]
- Muro, A.; Pérez-Arellano, J.-L. Nitric Oxide and Respiratory Helminthic Diseases. J. Biomed. Biotechnol. 2010, 2010, 958108. [Google Scholar] [CrossRef]
- Gomez-Flores, R.; Rodriguez-Padilla, C.; Mehta, R.T.; Galan-Wong, L.; Mendoza-Gamboa, E.; Tamez-Guerra, R. Nitric Oxide and TNF-Alpha Production by Murine Peritoneal Macrophages Activated with a Novel 20-KDa Protein Isolated from Bacillus thuringiensis Var. Thuringiensis Parasporal Bodies. J. Immunol. 1997, 158, 3796–3799. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Albina, J.E.; Cui, S.; Mateo, R.B.; Reichner, J.S. Nitric Oxide-Mediated Apoptosis in Murine Peritoneal Macrophages. J. Immunol. 1993, 150, 5080–5085. [Google Scholar] [CrossRef]
- Mandal, P.; Tewari, P.; Kumar, S.; Yadav, S.; Ayanur, A.; Chaturvedi, R.K.; Das, M.; Tripathi, A. Argemone Oil, an Edible Oil Adulterant, Induces Systemic Immunosuppression in Balb/c Mice in an Oral 28 Days Repeated Dose Toxicity Study. Chem. Biol. Interact. 2018, 287, 57–69. [Google Scholar] [CrossRef]
- Ochora, D.O.; Kakudidi, E.K.; Namukobe, J.; Ipulet, P.; Wakoli, D.M.; Okore, W.; Mwakio, E.W.; Yeda, R.A.; Cheruiyot, A.C.; Juma, D.W.; et al. Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae). Plants 2022, 11, 47. [Google Scholar] [CrossRef]




| Treatments | Abbreviation | Yield (%) |
|---|---|---|
| A. mexicana Ext. MeOH | AmexM | 17.63 |
| A. mexicana Fr. Hex | AmexHP | 3.43 |
| A. mexicana Fr. CHCl3 | AmexCP | 0.38 |
| A. mexicana Fr. MeOH | AmexMP | 1.86 |
| A. mexicana H2O | AmexAq | 11.96 |
| Berberine | BER | ¶ |
| Treatments | IC50 (µg/mL) in Cells | SI | IC50 (µg/mL) in Cells | SI | ||
|---|---|---|---|---|---|---|
| VERO | HEP-G2 | PBMC | L5178 Y-R | |||
| AmexM | 245.41 ± 13.05 c | 1020.77 ± 21.74 d | 0.24 a | 398.45 ± 8.01 b | 70.73 ± 2.40 b | 5.63 c |
| AmexHP | 120.36 ± 2.66 b | 45.48 ± 8.07 b | 2.64 c | >1200 | 155.21 ± 14.93 d | >7.70 d |
| AmexCP | 64.64 ± 5.18 a | 17.96 ± 1.59 a | 3.59 d | >1200 | 95.90 ± 3.19 c | >10.00 e |
| AmexMP | 380.78 ± 12.91 d | 459.87 ± 6.39 c | 0.83 b | >1200 | 573.83 ± 21.87 e | >2.00 b |
| AmexAP | 550.07 ± 17.12 e | 1156.19 ± 18.62 e | 0.32 a | 1173.15 ± 74.90 c | 1094.06 ± 96.03 f | 1.07 a |
| BER | 908.17 ± 31.86 f | 56.86 ± 9.45 b | 15.97 e | 27.14 ± 7.16 a | <5.0 a | >5.40 c |
| p—ANOVA | <0.01 | <0.001 | <0.001 | <0.05 | <0.001 | <0.01 |
| Treatment | Hemolytic Activity | Anti-Hemolytic Activity |
|---|---|---|
| IC50 (µg/mL) in Erythrocytes | ||
| AmexM | 973.88 ± 38.46 b | 32.85 ± 11.21 a |
| AmexHP | 3479.80 ± 236.19 e | 79.93 ± 4.22 b |
| AmexCP | 2163.63 ± 214.76 c | 1359.79 ± 116.10 d |
| AmexMP | 5309.10 ± 131.17 f | 73.04 ± 10.33 b |
| AmexAP | 2924.24 ± 125.71 d | 259.01 ± 31.73 c |
| BER | 712.74 ± 37.98 a | 36.88 ± 5.49 a |
| p—ANOVA | <0.001 | <0.001 |
| Treatments | A. salina | DPPH | ABTS | FRAP |
|---|---|---|---|---|
| LD50 in µg/mL | IC50 in µg/mL | IC50 in µg/mL | IC50 in µmol Fe2+/mL | |
| AmexM | 570.65 ± 11.19 c,*** | 565.98 ± 17.60 c | 158.99 ± 5.65 c | 751.82 ± 47.93 b |
| BER | 178.00 ± 29.70 b,** | 44.80 ± 1.22 a,* | 40.29 ± 9.02 a,* | 10.27 ± 2.04 a |
| Vitamin C | − | 68.90 ± 3.11 b | 81.76 ± 6.30 b | − |
| K2Cr2O7 | 29.44 ± 4.61 a | − | − | − |
| p—ANOVA | <0.001 | <0.01 | <0.01 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizondo-Luevano, J.H.; Quintanilla-Licea, R.; Monroy-García, I.N.; Kačániová, M.; Castillo-Velázquez, U.; Bazaldúa-Rodríguez, A.F.; Garza-Vega, L.M.; Torres-Hernández, Á.D.; Chávez-Montes, A. Assessment of Anticancer Properties of Argemone mexicana L. and Berberine: A Comparative Study. Plants 2024, 13, 1374. https://doi.org/10.3390/plants13101374
Elizondo-Luevano JH, Quintanilla-Licea R, Monroy-García IN, Kačániová M, Castillo-Velázquez U, Bazaldúa-Rodríguez AF, Garza-Vega LM, Torres-Hernández ÁD, Chávez-Montes A. Assessment of Anticancer Properties of Argemone mexicana L. and Berberine: A Comparative Study. Plants. 2024; 13(10):1374. https://doi.org/10.3390/plants13101374
Chicago/Turabian StyleElizondo-Luevano, Joel H., Ramiro Quintanilla-Licea, Imelda N. Monroy-García, Miroslava Kačániová, Uziel Castillo-Velázquez, Aldo F. Bazaldúa-Rodríguez, Lourdes M. Garza-Vega, Ángel D. Torres-Hernández, and Abelardo Chávez-Montes. 2024. "Assessment of Anticancer Properties of Argemone mexicana L. and Berberine: A Comparative Study" Plants 13, no. 10: 1374. https://doi.org/10.3390/plants13101374








